Agent kit containing oral insulin solid therapy
A kit and insulin technology, which can be used in medical preparations containing active ingredients, metabolic diseases, peptide/protein components, etc., and can solve problems such as interference and hypoglycemia.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0164] Preparation of delivery agent 4-CNAB
[0165] Compounds corresponding to the following structures were prepared as described below:
[0166]
[0167] 4-Chlorosalicylic acid (10.0 grams, 0.0579 moles) was added to a 250 ml single necked round bottom flask containing about 50 ml of dichloromethane. Stirring was started and continued for the remainder of the reaction. The coupling agent 1,1-carbonyldiimidazole (9.39 g, 0.0579 mol) was added to the flask as a solid portion. After adding all the coupler, stir at room temperature for about 20 minutes, then add ethyl-4-aminobutyric acid hydrochloride (9.7 g, 0.0579 mol) with stirring. Then, triethylamine (10.49 ml, 0.0752 mol) was added dropwise through a funnel. The funnel was rinsed with dichloromethane. The reaction was stirred overnight at room temperature.
[0168] The reactant was poured into a separatory funnel and washed with 2 N hydrochloric acid to form an emulsion. The emulsion was allowed to stand for 2 da...
example 2
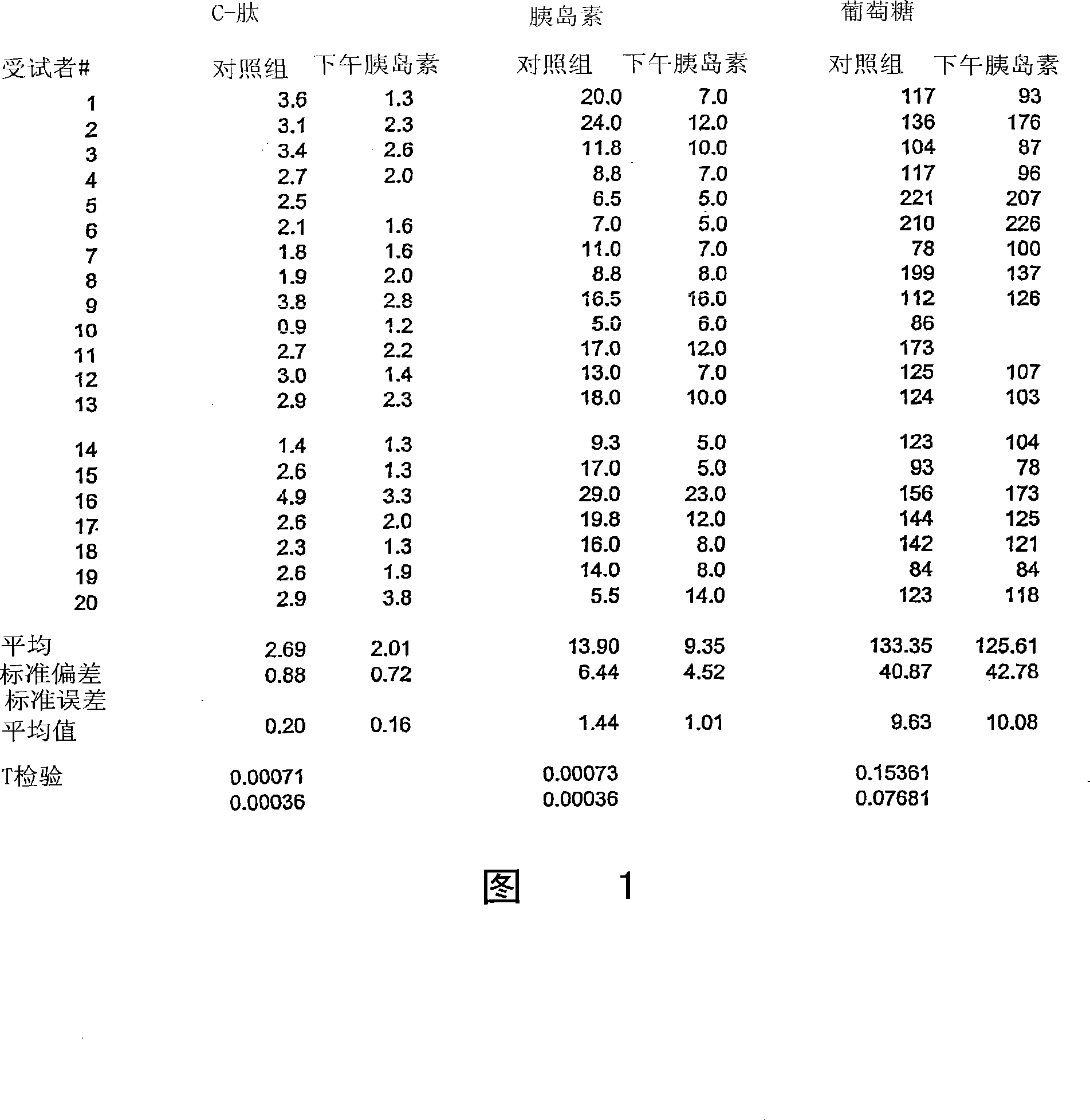
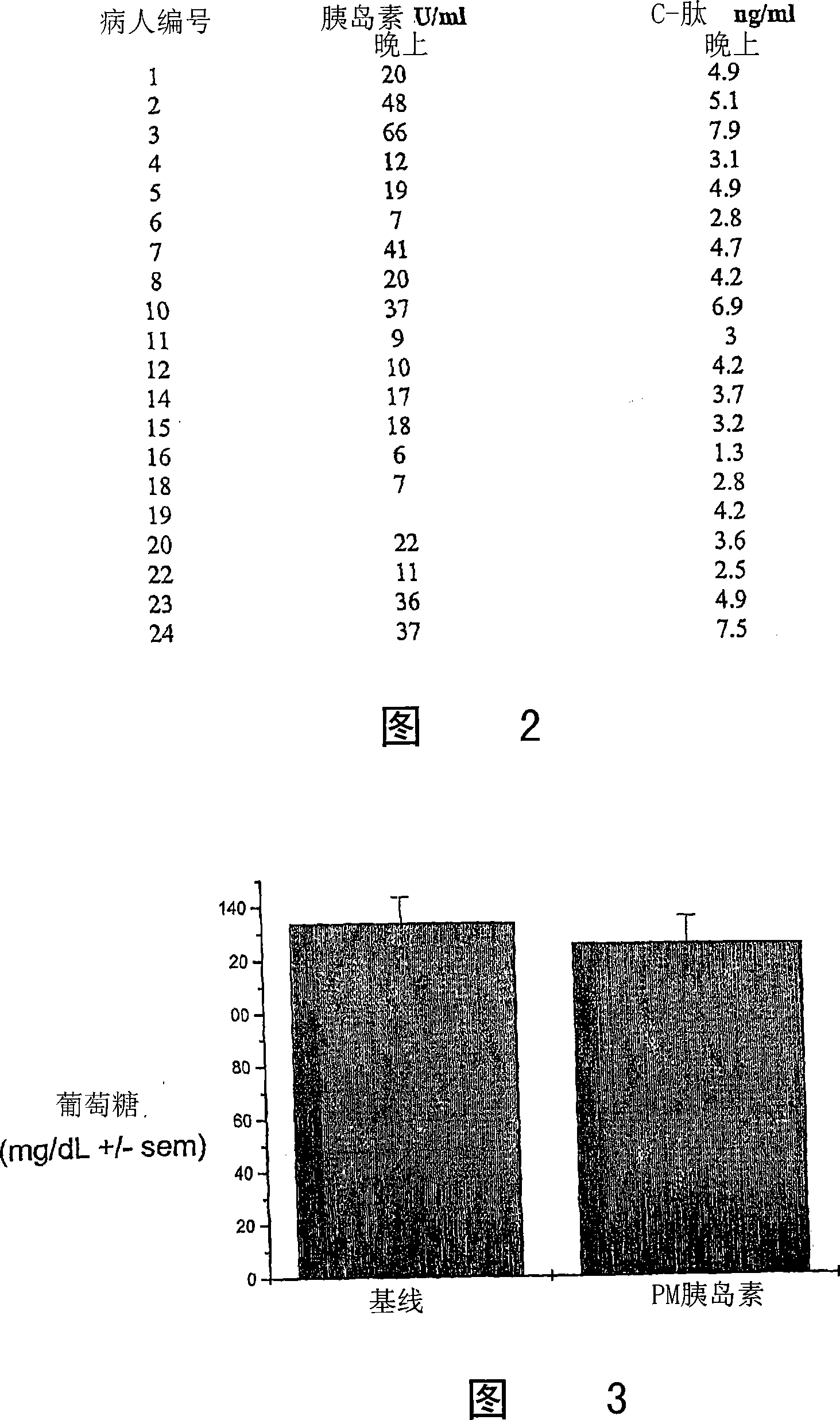
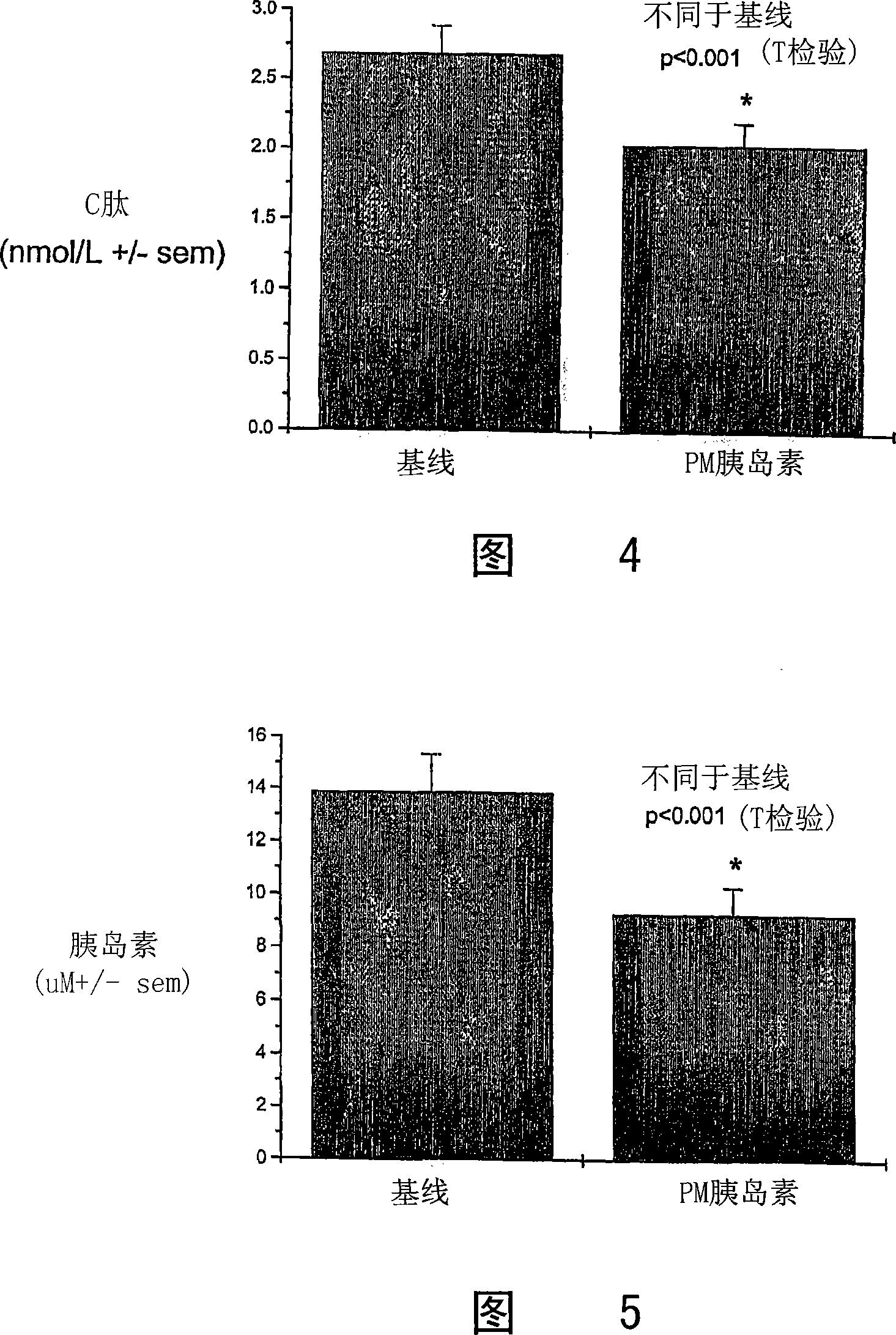
[0179] Nonclinical studies of 4-CNAB and insulin / 4-CNAB
[0180] For the composition of the present invention comprising insulin and delivery agent 4-CNAB, the safety and toxicity in non-clinical programs were evaluated, including pharmacological screening, pharmacokinetic distribution and toxicity evaluation in mice and monkeys. In general, the physiological responses of animals to 4-CNAB alone were comparable to those to insulin / 4-CNAB. Pharmacokinetic studies in mice, rats and monkeys have shown that 4-CNAB is rapidly absorbed after oral administration and then cleared from the body. In receptor binding screening assays, 4-CNAB did not show potential activity at any of the primary molecular targets. Four genotoxicity studies conducted with 4-CNAB were not positive. Based on a 14-day oral repeated dose toxicity study, the NOAEL (No Adverse Effect Level) was estimated to be 500 mg / kg in Sprague-Dawley rats and 400 mg / kg in rhesus monkeys.
[0181] In toxicity studies, dose...
example 3
[0185] This example describes the method for preparing insulin / 4-CNAB capsules. 4-CNAB prepared as above was first screened through a 35 mesh. Weigh the desired amount of screened 4-CNAB and place in a covered weighing pan. Weigh the required amount of insulin into the covered weighing pan.
[0186] The above insulin was sifted through a 35 mesh onto the same mortar and approximately 2.0 grams of 4-CNAB was sifted through the same 35 mesh onto the insulin. Gently grind the contents of the mixing mortar with a glass mortar and pestle for 3 minutes, scraping with a spatula if necessary. The above 4-CNAB was continued through a 35 mesh screen in a small fraction corresponding to the volume of the material in the mortar. The contents of the mortar were mixed for approximately 3 minutes after each addition.
[0187] After the final addition, gently grind the contents of the mixing mortar with a glass mortar and pestle for 3 minutes, scraping with a spatula if necessary. The fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com