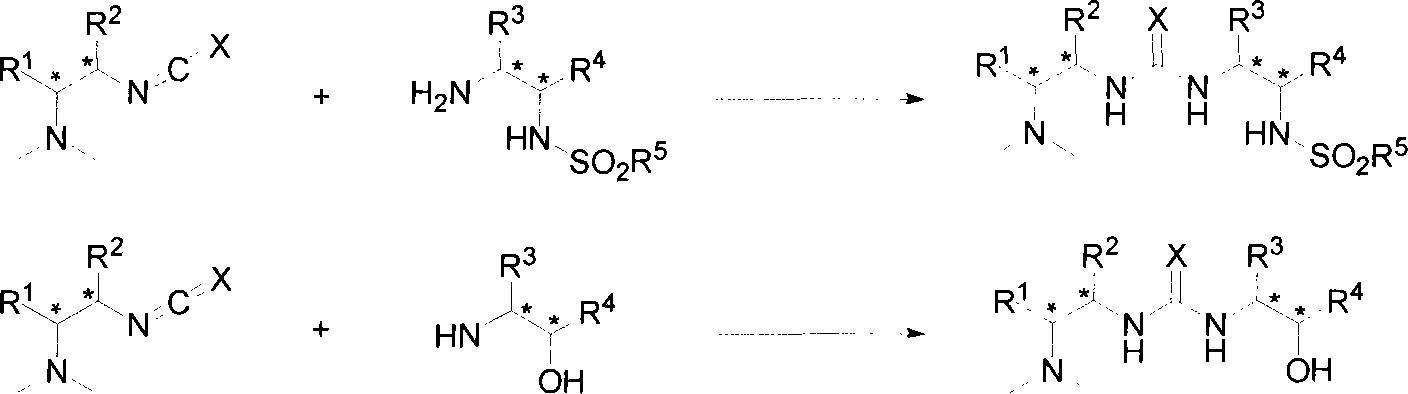
Chiral compound having multi-hydrogen bonds dual-function as well as synthetic method and use thereof
A chiral compound and dual-functional technology, which is applied in organic chemical methods, preparation of organic compounds, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of high catalyst usage and limited substrate application range, etc. problem, to achieve the effect of fast catalytic reaction speed, low catalyst dosage and good enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of catalyst: 1-((1R, 2R)-2-(dimethylamino)cyclohexyl)-3-((1R, 2R)-1,2-diphenyl-2-(methyl Sulfonamide) ethyl) thiourea
[0021]
[0022] Add (1R,2R)-2-isothiocyanato-N,N-dimethylcyclohexylamine (24mg, 0.13mmol) to a solution of (1R,2R)-1,2-diphenyl A solution of N-methylsulfonyl-1,2-ethylenediamine (37 mg, 0.13 mmol) in dry tetrahydrofuran (1 mL) was stirred at room temperature for 12 hours. After distilling the solvent to dryness, the mixture was subjected to silica gel column chromatography to obtain the product. Yield 97%. Mp 240-241°C; [α] 25 D +9.4° (c0.44, CHCl 3 ); IR (KBr) 3400, 1675, 1595, 1500, 1445, 1270, 1040, 965, 670cm-1; 1 H NMR (CDCl 3 , TMS, 300MHz) δ1.21-1.24(m, 4H), 1.69-1.90(m, 4H), 2.20(m, 1H), 2.41(s, 6H), 2.47(s, 3H), 3.70(m, 1H), 4.79(d, J=10.2Hz, 1H), 5.88(s, 1H), 7.08-7.22(m, 10H); 13 C NMR (CDCl 3 , TMS, 75MHz) δ22.65, 24.37, 24.52, 32.59, 39.86, 41.69, 55.24, 62.25, 64.38, 66.34, 127.64, 127.76, 128.44, 128....
Embodiment 2
[0023] Example 2: Preparation of catalyst: 1-((1R, 2R)-2-(dimethylamino)cyclohexyl)-3-((1S, 2S)-1,2-diphenyl-2-(p Tosylamide) ethyl) thiourea
[0024]
[0025] Add (1R,2R)-2-isothiocyanato-N,N-dimethylcyclohexylamine (40.23mg, 0.22mmol) to a solution of (1S,2S)-1,2-di A solution of phenyl-N-p-toluenesulfonyl-1,2-ethylenediamine (120 mg, 0.33 mmol) in dry THF (2 mL) was stirred at room temperature for 12 hours. After distilling the solvent to dryness, the mixture was subjected to silica gel column chromatography to obtain the product. Yield 85%. Mp.110-113℃; [α] 25 D +18.5 (c 0.62, CHCl 3 ); IR(KBr)v 3357, 3062, 3030, 2932, 2858, 1536, 1327, 1155cm -1 ; 1 H NMR (CDCl 3 , TMS, 300MHz) δ1.08-1.29(m, 4H), 1.63-1.76(m, 3H), 1.95(s, 6H), 2.20(m, 1H), 2.25(s, 3H), 2.42(m, 1H), 3.58(m, 1H), 4.70(d, J=10.2Hz, 1H), 5.68(m, 1H), 6.86-7.13(m, 12H), 7.41(d, J=8.1Hz, 2H); 13 C NMR (CDCl 3 , TMS, 75MHz) δ21.59, 22.37, 24.79, 25.17, 33.41, 40.13, 56.67, 62.99, 63.84, 67.25, 127....
Embodiment 3
[0026] Example 3: Preparation of catalyst: 1-((1R, 2R)-2-(dimethylamino)cyclohexyl)-3-((1R, 2R)-1,2-diphenyl-2-(p Tosylamide) ethyl) thiourea
[0027]
[0028] Add (1R,2R)-2-isothiocyanato-N,N-dimethylcyclohexylamine (40.23mg, 0.22mmol) to a solution of (1R,2R)-1,2-di A solution of phenyl-N-p-toluenesulfonyl-1,2-ethylenediamine (80 mg, 0.22 mmol) in dry THF (2 mL) was stirred at room temperature for 12 hours. After distilling the solvent to dryness, the mixture was subjected to silica gel column chromatography to obtain the product. Yield 80%. Mp.240-241℃; [α] 25 D +3.5°(c0.62, CHCl 3 ); IR (KBr) 3400, 1675, 1595, 1500, 1445, 1270, 1040, 965, 670cm-1; 1 HNMR (CDCl 3 , TMS, 300MHz) δ1.18-1.27(m, 4H), 1.68-1.90(m, 3H), 2.30(s, 1H), 2.35(s, 6H), 2.20-2.43(m, 2H), 3.47( s, 1H), 4.72(d, J=10.8Hz, 1H), 5.81(m, 1H), 6.86-7.21(m, 12H), 7.40(d, J=10.8Hz, 2H); 13 C NMR (CDCl 3 , TMS, 75MHz) δ21.67, 22.88, 24.76, 25.11, 32.89, 41.29, 56.85, 63.04, 64.79, 67.17, 126.99, 127.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com