Method for synthesizing mu-oxo binuclear ferriporphyrin
A technology of iron porphyrin and oxobi is applied in iron organic compounds, organic chemistry and other directions, which can solve the problems of serious environmental pollution, long metallization time, difficult separation and purification, etc., to reduce pollution, shorten reaction time, and facilitate purification. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
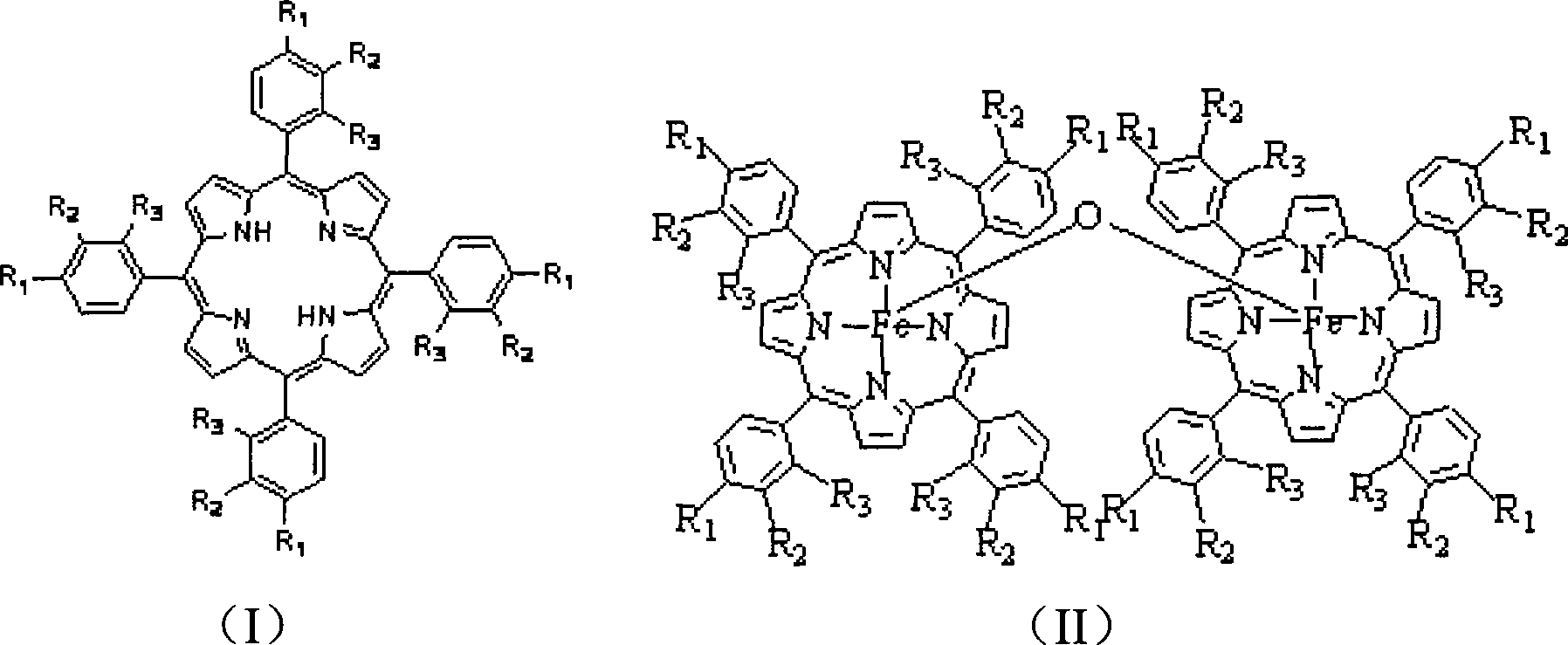
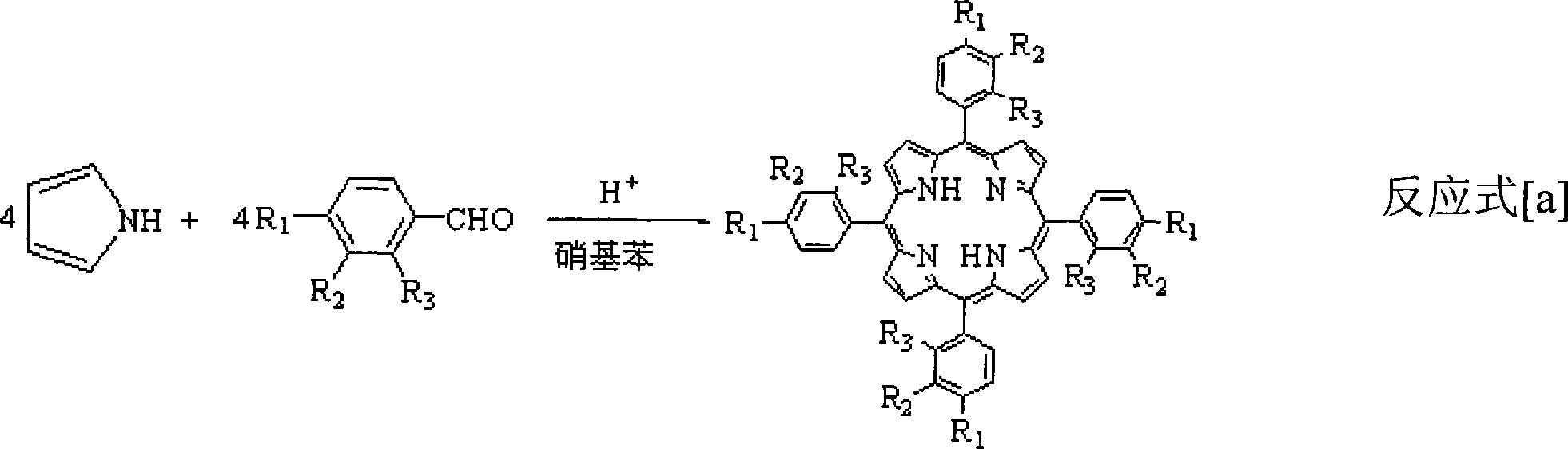
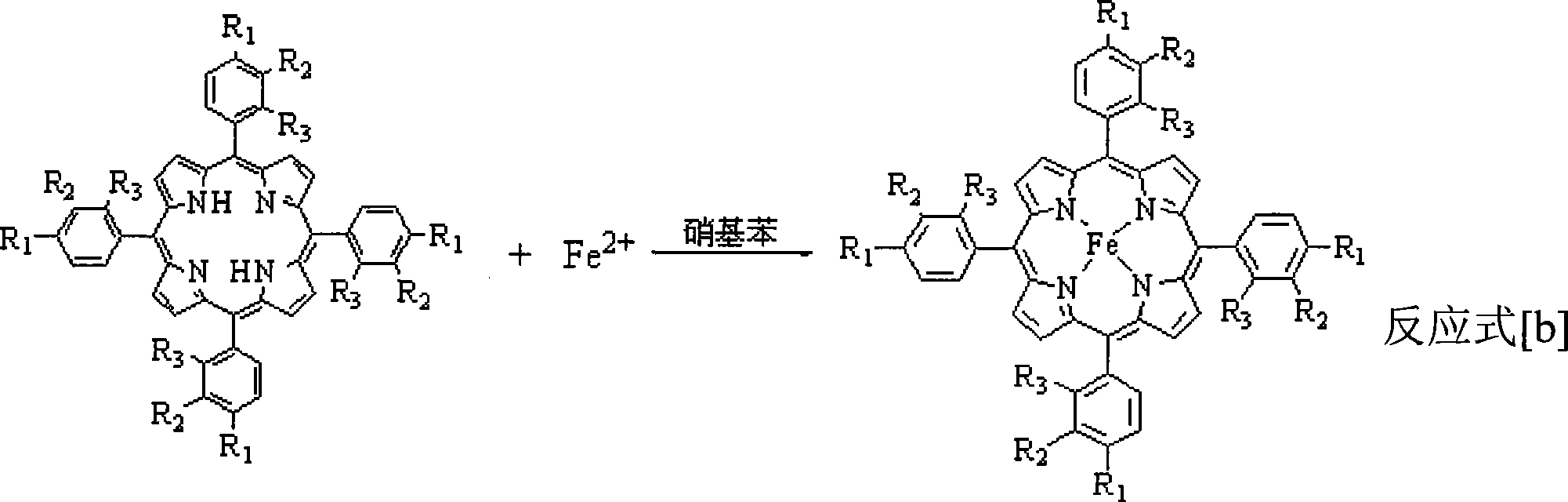
[0028] In a 250mL three-necked flask, add a mixed solvent of 1mL acetic acid and 99mL DMF (both volume percentages are 1% and 99%, respectively), add 1mmol porphyrin ligand and 1mmol ferrous sulfate, heat and reflux for 5min, cool to room temperature and add 20mL water, suction filtration, wash filter cake with ethanol and water, dry, obtain μ-oxo binuclear iron porphyrin (being R in general formula (II) 1 = H, R 2 = H, R 3 =H), the yield was 95%.
Embodiment 2
[0030] In a 250mL three-necked flask, add a mixed solvent of 20mL acetic acid, 10mL caprylic acid and 70mL DMF (the three volume percentages are 20%, 10%, 70%, respectively), add 1mmol p-methylporphyrin ligand and 1.5mmol ferrous acetate , heating to reflux for 0.5min, cooling to room temperature, adding 5mL of water, suction filtration, washing the filter cake with ethanol and water, and drying to obtain μ-oxo-p-methylbinuclear iron porphyrin (i.e. R in the general formula (II) 1 =CH 3 , R 2 = H, R 3 =H), the yield was 97%.
Embodiment 3
[0032] In a 250mL three-neck flask, add 50mL propionic acid, 50mL DMF (50% and 50% by volume respectively) mixed solvent, add 1mmol o-chloroporphyrin ligand and 3mmol ferrous acetate, heat to reflux for 1min, and cool to room temperature Then add 50mL water, suction filter, wash the filter cake with ethanol and water, and dry to obtain μ-oxo-o-chlorobinuclear iron porphyrin (i.e. R in the general formula (II) 1 = H, R 2 = H, R 3 =Cl), the yield was 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com