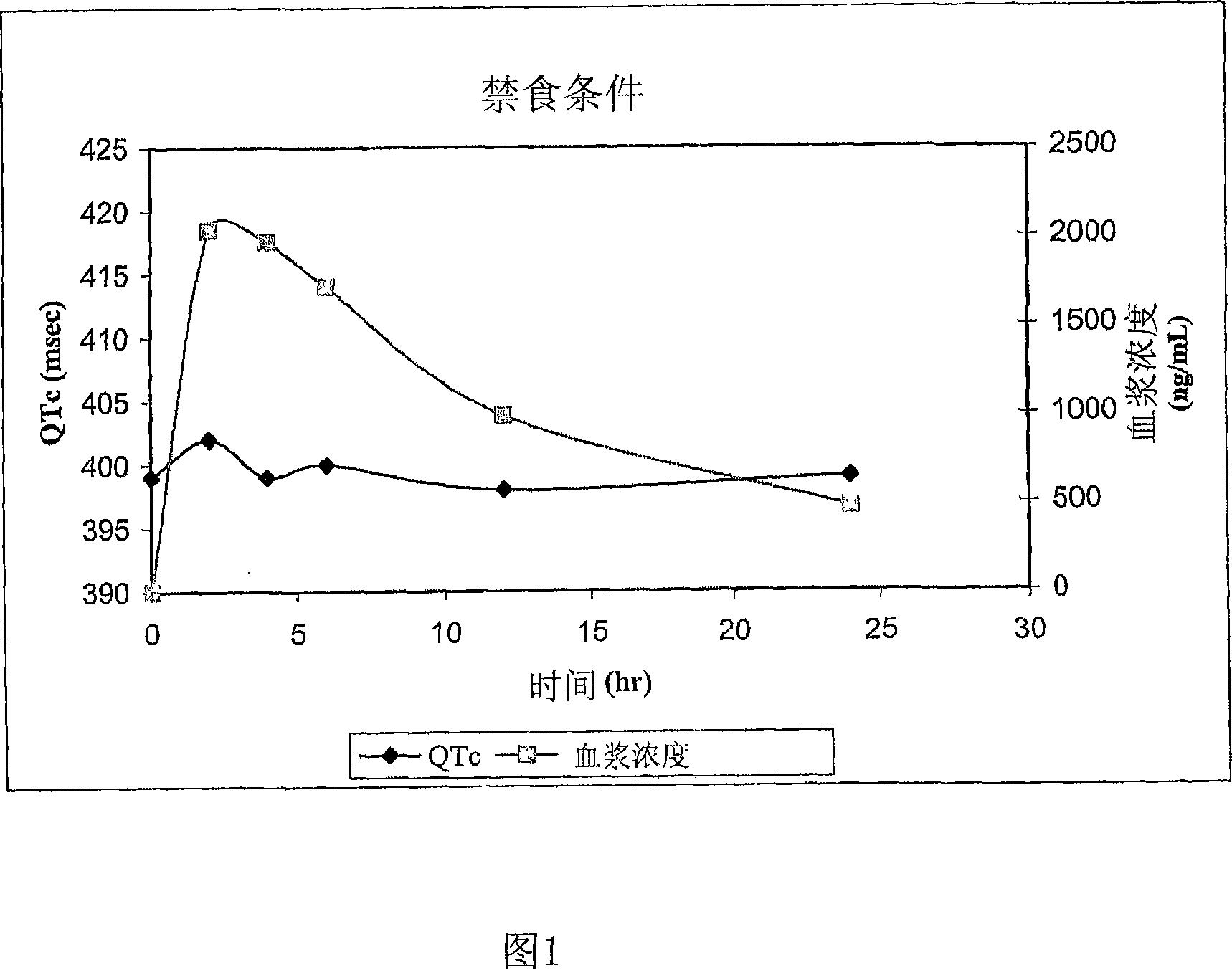
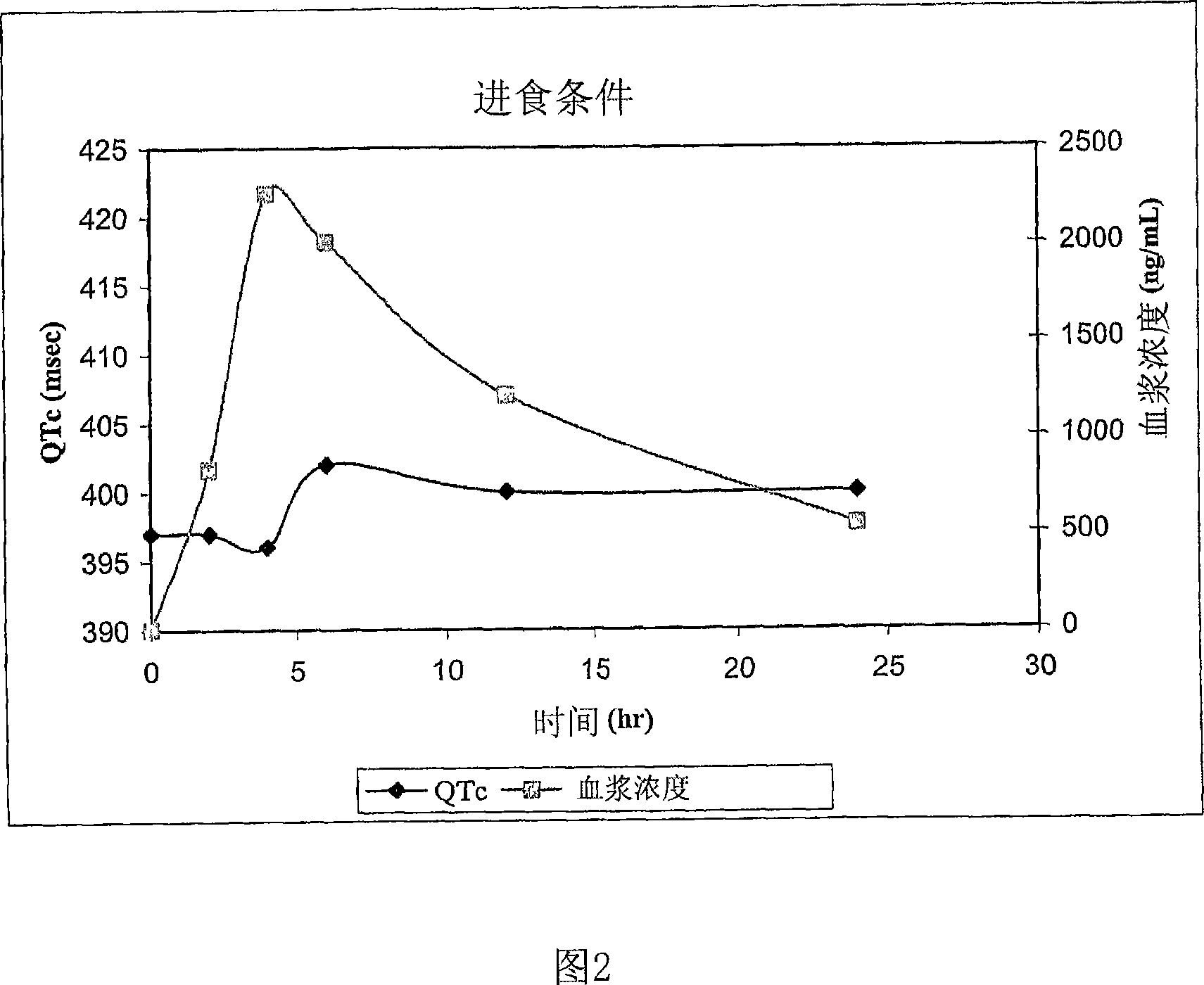
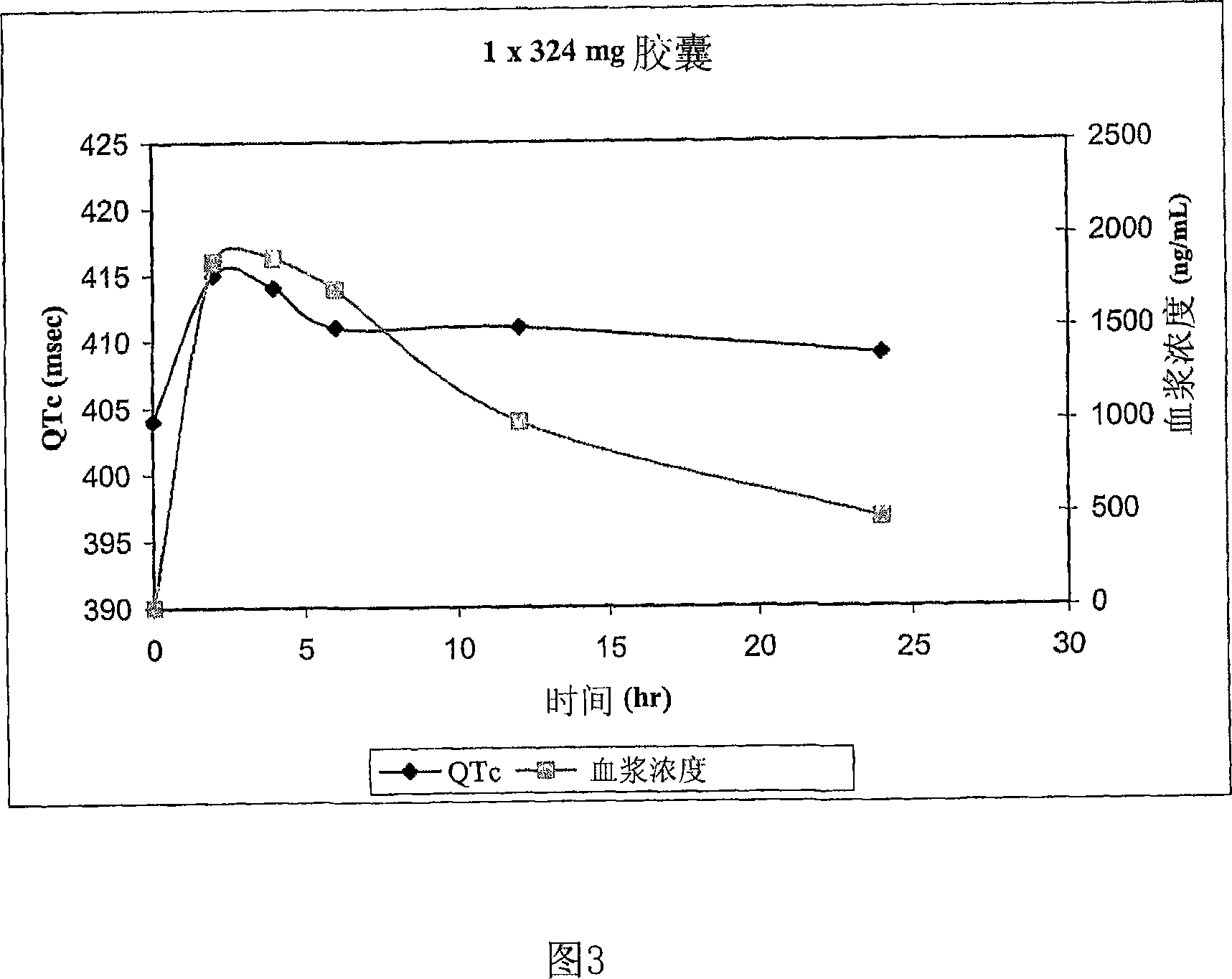
Quinine-containing controlled-release formulations
A technology for controlled-release preparations and preparations, which can be used in pill delivery, ICT adaptation, active ingredients of heterocyclic compounds, etc., and can solve problems such as QT prolongation will not be reached.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0147] The preparation steps of the support layer components are: mixing, if possible, wetting with a binding solution in a known field, and then making the mixture into a dry granular shape. The mixture can be sieved and then mixed with other components to obtain an easy-flowing homogeneous mixture. The prepared support layer mixture is coated on the core and pressed into a surface layer. The support layer may be coated on one or two base surfaces of the core, or on the entire core surface except for one base surface, or on the entire side surface except for the two base surfaces. The support layer is usually about 1000-4000kg / cm 2 The pressure is overwhelming.
[0148] In one embodiment, the sustained-release preparation comprises (a) a deposit-core, which has a defined geometric shape, contains a therapeutically effective amount of quinine or a pharmaceutically acceptable salt thereof, and a core polymer material, the core polymer material is selected from (1) Expansive polymer...
Embodiment 1
1000
1200
[0194] The ingredients other than the lubricant are mixed in a high-shear granulator. Water is added, the wet mixture is granulated, the granules are sieved, dried and ground. Then add the granules into the low-shear mixer, add lubricant and stir. The final blend is compressed in a tablet press to form a sustained-release quinine dosage form.
Embodiment 2
[0195] The ingredients other than lubricant and flow agent are mixed in a low shear mixer for 20 minutes. Add lubricant and flow agent and stir for 5 minutes. Press directly in the tablet press.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com