Hyperbranched poly-fluorene material containing space steric hindrance group and manufacture method thereof
A technology of hyperbranched polymerization and steric hindrance, applied in chemical instruments and methods, luminescent materials, semiconductor/solid-state device manufacturing, etc., can solve problems such as limited solubility, achieve the effect of improving spectral thermal stability and inhibiting molecular aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
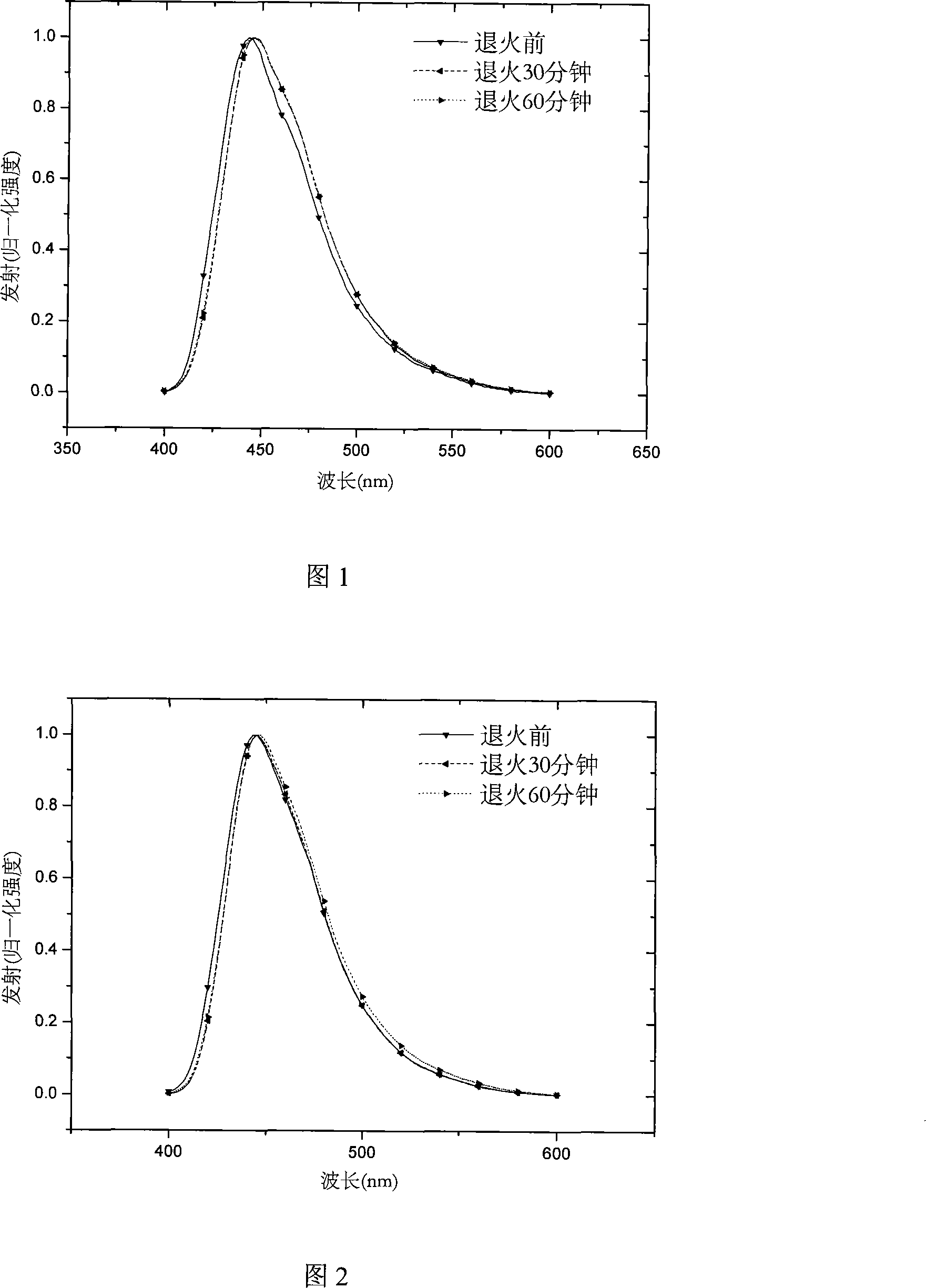
[0020] The intermediates 2,7-dibromo-9,9 dioctylfluorene and 2,7-diboronic acid ester-9,9 dioctyl fluorene were prepared by the following method, and the end-capping agent 2-boronic acid was prepared by a similar method lipid-9,9-dioctylfluorene. 2,7-dibromo-9,9-dioctylfluorene, 2,7-diboronic acid ester-9,9-dioctylfluorene, 1,3,5-m-tribromobenzene and 9,10-dibromoanthracene The molar ratio of 29:43:4:8 is fed, and after Suzuki reaction for 60 to 72 hours, a certain proportion of 2-boronic acid ester-9,9 dioctylfluorene and catalyst tetrakis(triphenylphosphine)palladium (0 ), continue to react, through separation and purification, obtain hyperbranched polymer I, and its reaction scheme is as follows:
[0021]
[0022]
[0023]
[0024]
Embodiment 2
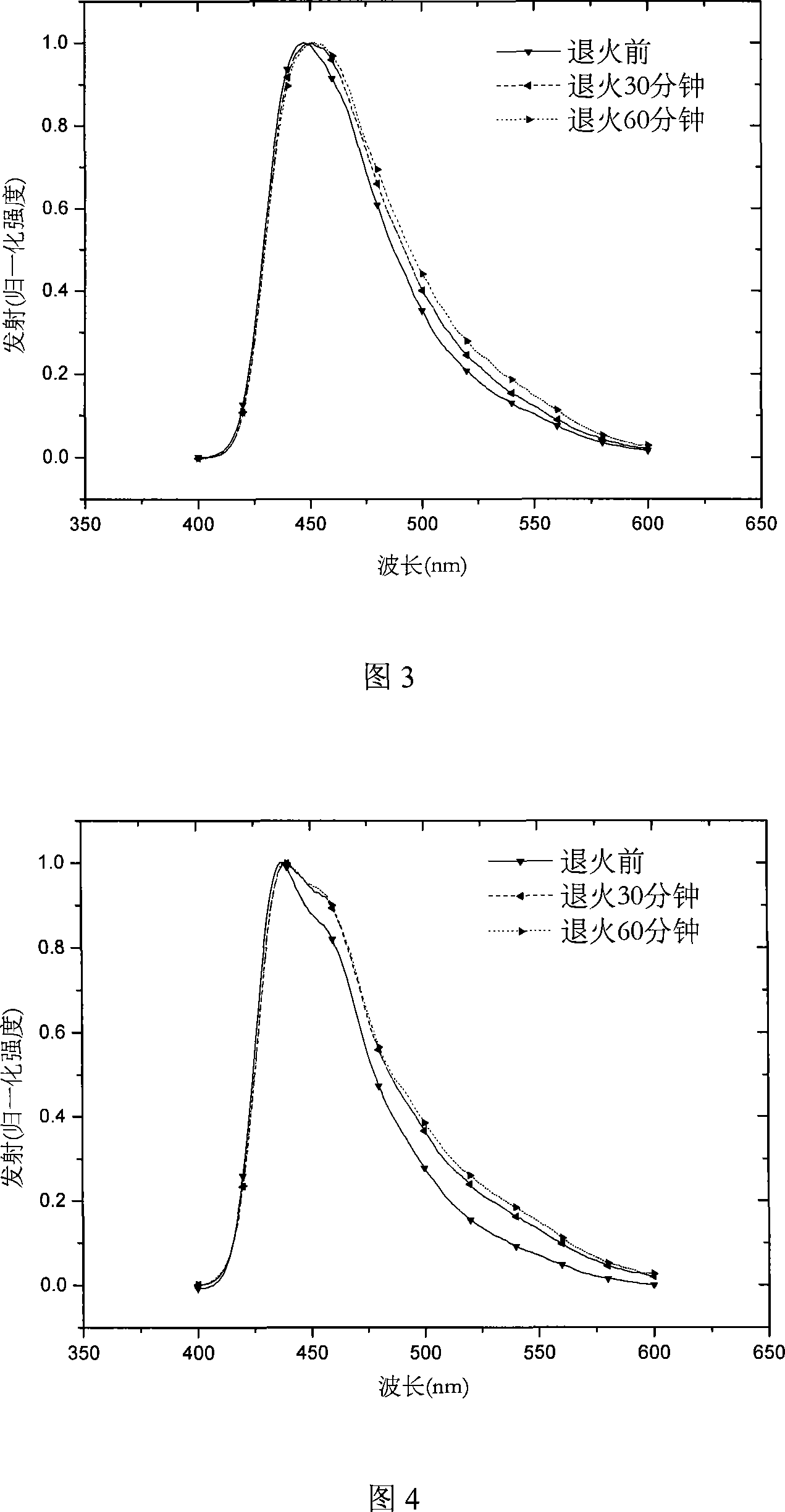
[0026] Prepare intermediate 2,7-dibromo-9,9 dioctyl fluorene, 2,7-diboronic acid ester-9,9 dioctyl fluorene and 2,7-dibromo-spiro( Fluorene-9,9-oxanthene), and a similar method was used to prepare the end-capping agent 2-boronic acid ester-9,9 dioctylfluorene. 2,7-dibromo-9,9-dioctylfluorene, 2,7-diboronic ester-9,9-dioctylfluorene, 1,3,5-m-tribromobenzene and 2,7-dibromo-spiro (Fluorene-9,9-oxanthene) is charged at a molar ratio of 29:43:4:8, and after Suzuki reaction for 60 to 72 hours, a certain proportion of 2-boronic acid ester-9,9 dioctylfluorene and Catalyst four (triphenylphosphine) combined palladium (0), continue to react, through separation and purification, obtain hyperbranched polymer II, its reaction scheme is as follows:
[0027]
[0028]
[0029]
[0030]
[0031]
Embodiment 3
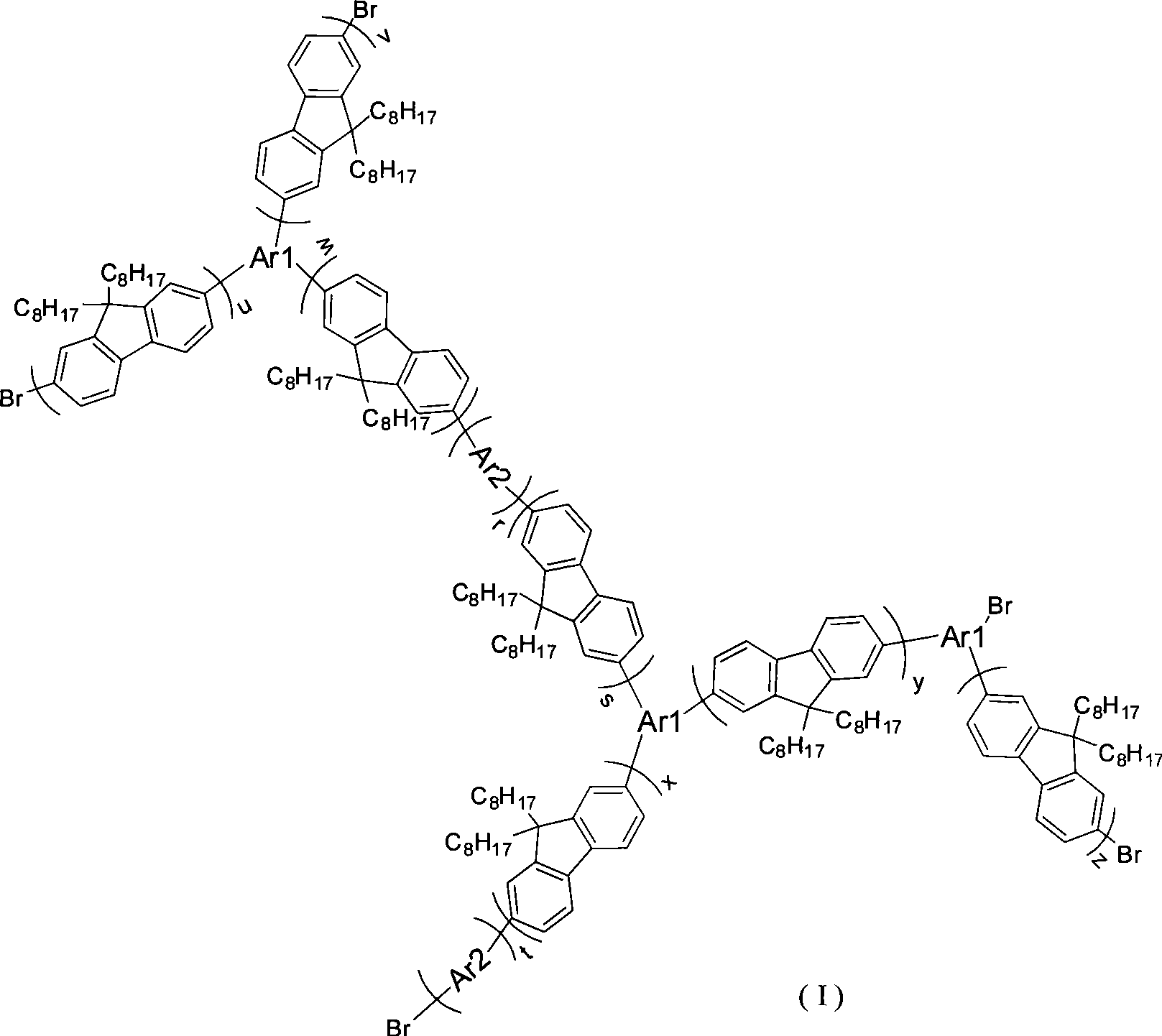
[0033] Prepare intermediate 2,7-dibromo-9,9 dioctyl fluorene and 2,7-diboronic acid ester-9,9 dioctyl fluorene by the following method as in Example 1, and obtain end-capped Agent 2-boronate-9,9 dioctylfluorene. 2,7-dibromo-9,9 dioctylfluorene, 2,7-diboronic acid ester-9,9 dioctyl fluorene, tris(4-bromophenyl)amine and 9,10-dibromoanthracene 13 : 23: 4: 4 molar ratio feeding, after Suzuki reaction for 60 to 72 hours, a certain proportion of 2-boric acid ester-9,9 dioctyl fluorene and catalyst tetrakis (triphenylphosphine) palladium (0) , continue to react, through separation and purification, obtain hyperbranched polymer III, and its reaction scheme is as follows:
[0034]
[0035]
[0036]
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com