Transition metal complex compound and organic electroluminescent device using same
A technology of coordination compounds and transition metals, applied in luminescent materials, organic chemistry, and compounds containing elements of Group 8/9/10/18 of the periodic table, etc., can solve energy efficiency, low external quantum efficiency, no inspiration, There is no mention of issues such as long-life luminescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
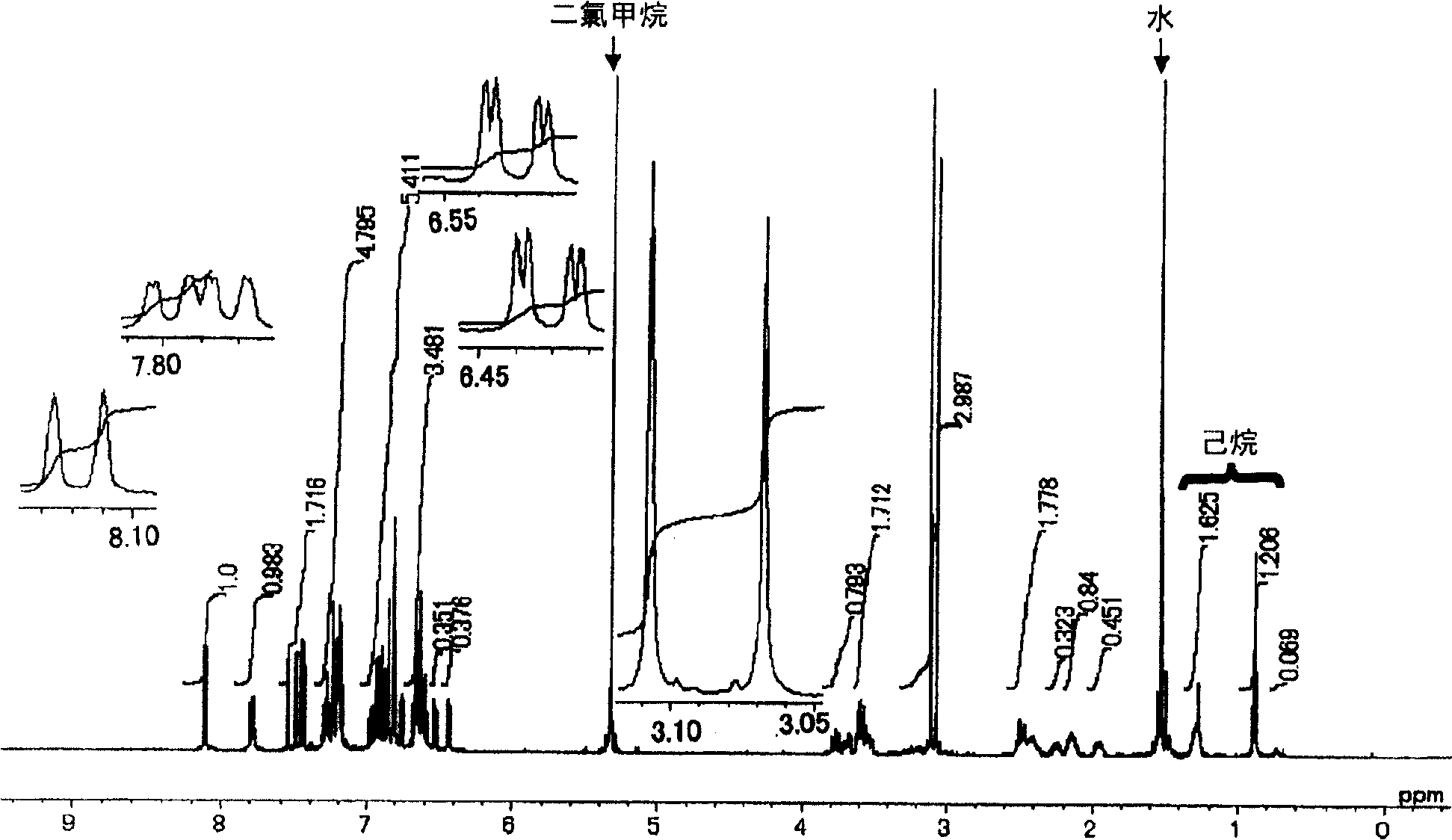
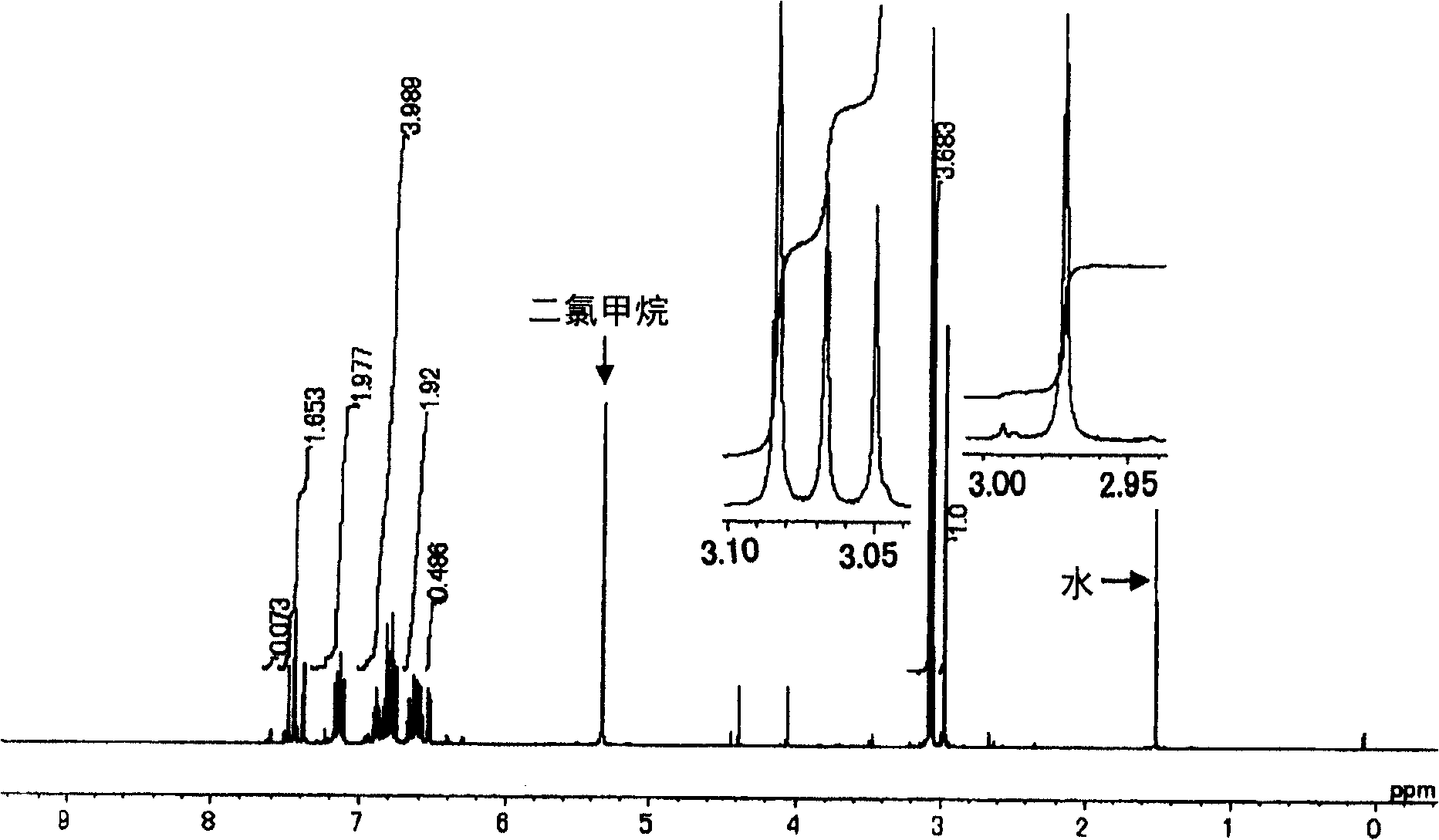
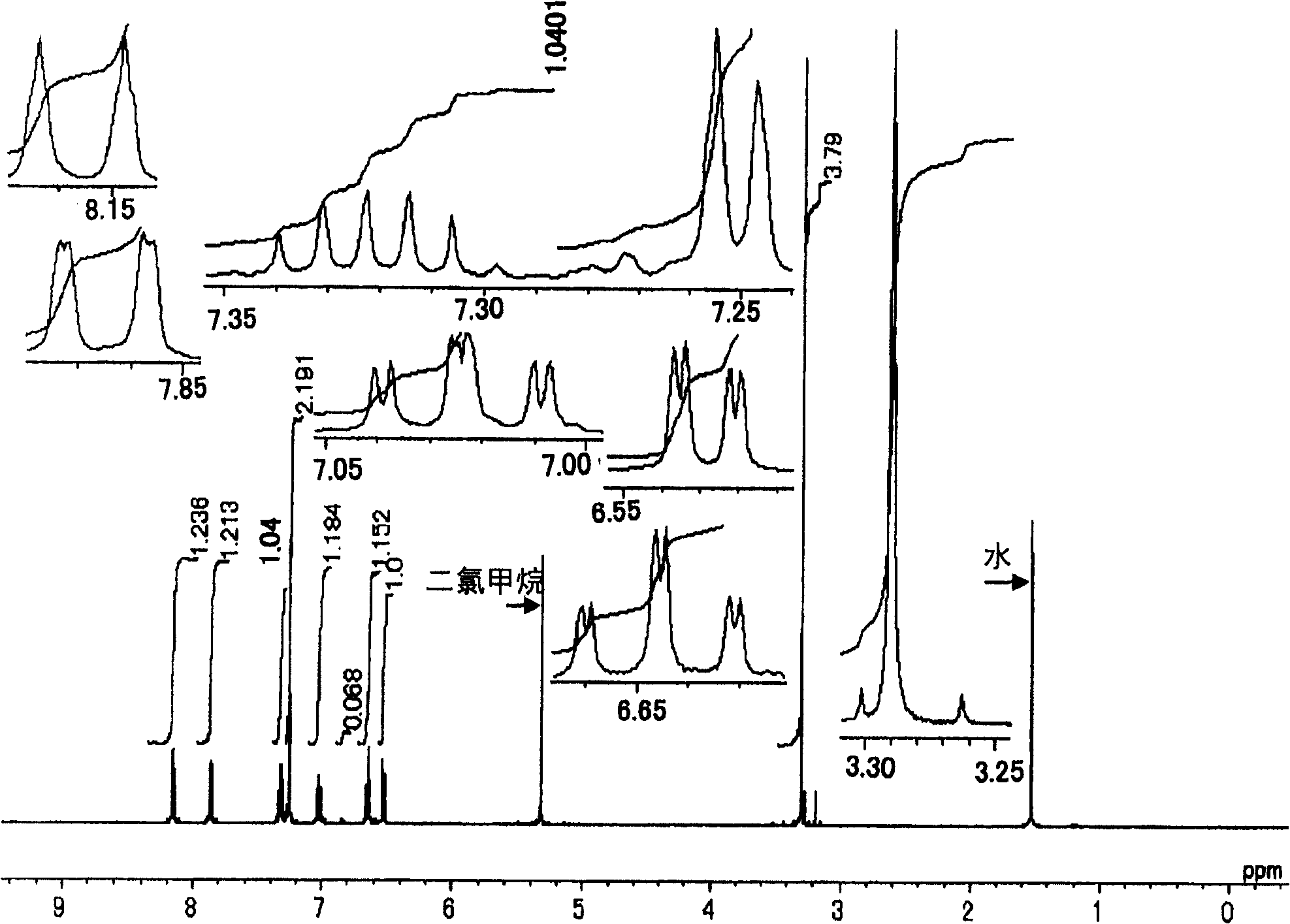
Image
Examples
Embodiment 1
[0334] Embodiment 1 (synthesis of transition metal coordination compound 1)
[0335] (i) Synthesis of cross-linked ligand precursor (compound a)
[0336] The crosslinking ligand precursor (compound a) was synthesized by the following reaction procedure.
[0337] [chem 44]
[0338]
[0339] Add 100ml tetrahydrofuran (THF) to 5.00g N-phenylimidazole (molecular weight 144.18, 34.7 mmol) and 5.05 g of 1,4-diiodobutane (molecular weight 309.92, 16.3 mmol), stir at room temperature for 8 Hour. The resulting white solid (compound a) was filtered off, and the filtrate was stirred for 8 hours (this operation was repeated twice) to obtain a total of 5.50 g of compound a (yield 56%).
[0340] (ii) Synthesis of ligand precursor (compound b)
[0341] The ligand precursor (compound b) was synthesized by the following reaction procedure.
[0342] [chem 45]
[0343]
[0344] To 9.21 g of N-phenylo-phenylenediamine (molecular weight 184.24, 50 mmol) was added 100 ml of toluene, fol...
Embodiment 2
[0416] Embodiment 2 (synthesis of transition metal coordination compound 2)
[0417] (i) Synthesis of the crosslinking group site (compound e)
[0418] Synthesize 1.25 g of the following compound e ( Molecular weight 336.89, 3.71 mmol).
[0419] [Chemical 52]
[0420]
[0421] (ii) Synthesis of ligand (compound f)
[0422] The ligand (compound f) was synthesized by the following reaction procedure.
[0423] [Chemical 53]
[0424]
[0425] 40 ml of tetrahydrofuran was added to 1.93 g of N-phenylimidazole (molecular weight 144.18, 13.4 mmol) and 1.25 g of compound e (molecular weight 336.89, 3.71 mmol), and refluxed for 8 hours. The resulting white solid was filtered off to obtain 1.19 g of compound f (molecular weight 769.41, 1.54 mmol, yield 32%).
[0426] (ii) Synthesis of transition metal complex 2
[0427] Transition metal complex 2 was synthesized by the following reaction procedure.
[0428] [Chemical 54]
[0429]
[0430] The entire reaction was carried...
Embodiment 3
[0432] Embodiment 3 (synthesis of transition metal coordination compound 3)
[0433] The transition metal complex 3 was synthesized by the following reaction procedure.
[0434] [Chemical 55]
[0435]
[0436] The entire reaction was carried out under argon flow. 21 ml of 2-ethoxyethanol was added as a solvent to 0.140 g of compound c (molecular weight 671.70, 0.209 mmol), followed by 0.142 g of sodium ethoxide (molecular weight 68.05, 2.09 mmol), and stirred at room temperature for 1 hour. 0.157 g of compound g (molecular weight 376.19, 0.418 mmol) was added thereto, followed by 0.250 g of compound a (molecular weight of 598.26, 0.418 mmol), and the reaction was carried out under reflux for 2 hours. From the obtained reaction solution, 2-ethoxyethanol as a solvent was distilled off by heating under reduced pressure, and the obtained solid was purified by silica gel column chromatography (developing solvent: dichloromethane, Rf value about 0.8). As a result, 0.013 g of c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com