Method for processing nickel bearing raw material in chloride-based leaching
A chloride, leaching technology, applied in the direction of improving process efficiency, can solve problems such as large production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
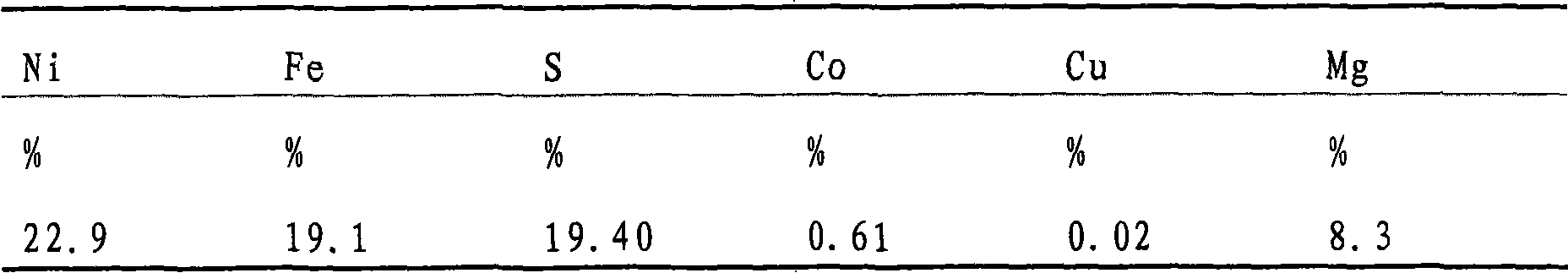
[0079] The nickel sulfide concentrate is leached according to the method of the present invention. The concentrate contains a major part of the nickel bound in the pentlandite form. Other major minerals are serpentinite, goethite and pyrite. The chemical analysis of the concentrate is:
[0080]
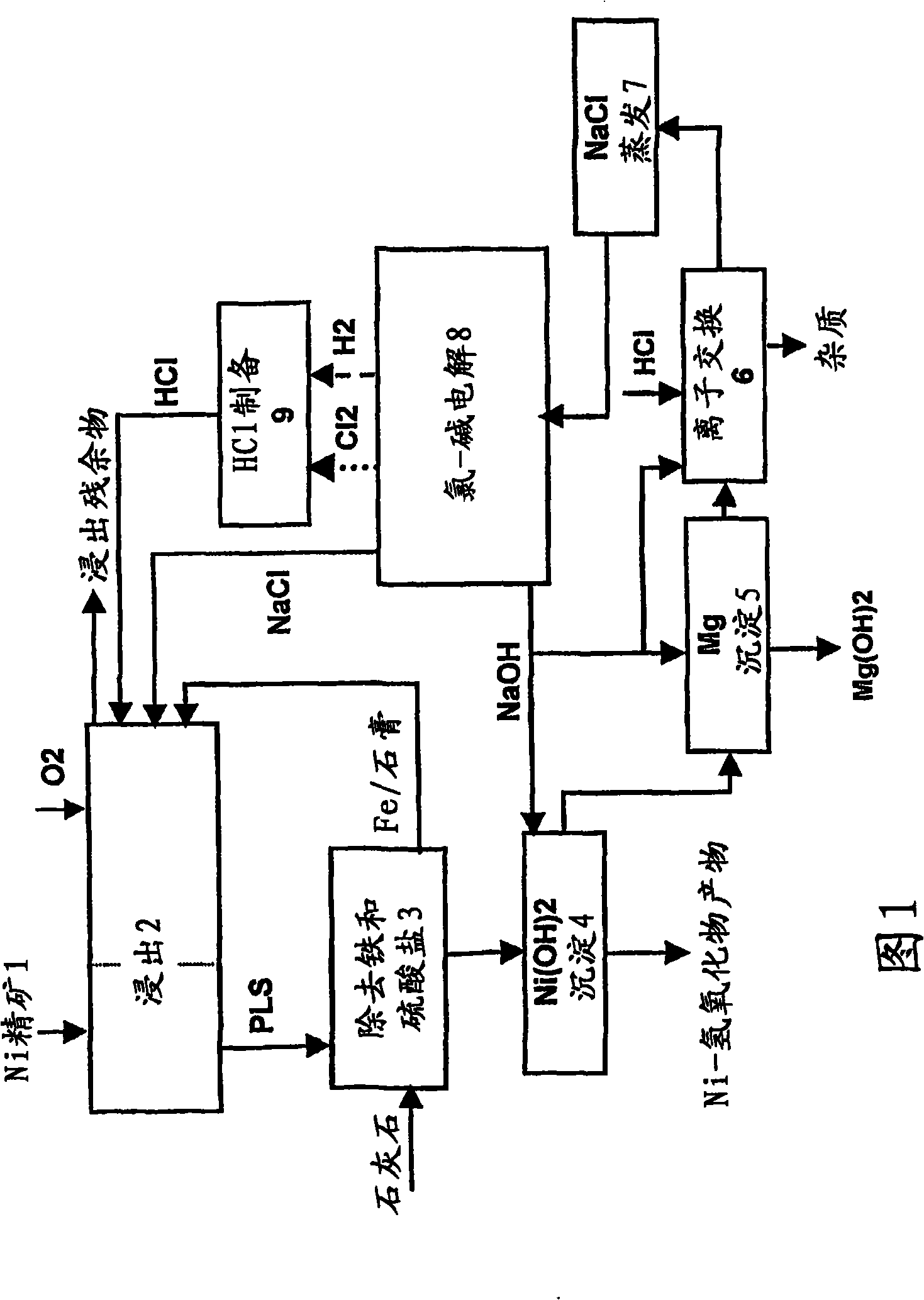
[0081] Leaching step 1
[0082] Nickel concentrate (1000g) was leached in solution (3170ml) with an initial content of:
[0083]
[0084] No oxidizing agent is used in leaching, and the pH of the solution is 2-3 during leaching. Copper from solution as chalcocite Cu 2 The S form precipitates while the nickel dissolves. After three hours of leaching at a temperature of 95° C., a solution (leaching product solution, PLS) was obtained, the composition of which was:
[0085]
[0086] After three hours, the solid composition was:
[0087]
[0088] Leaching step 2
[0089] The solid, i.e. the solid leaching residue after iron removal in the first leaching step, is oxidat...
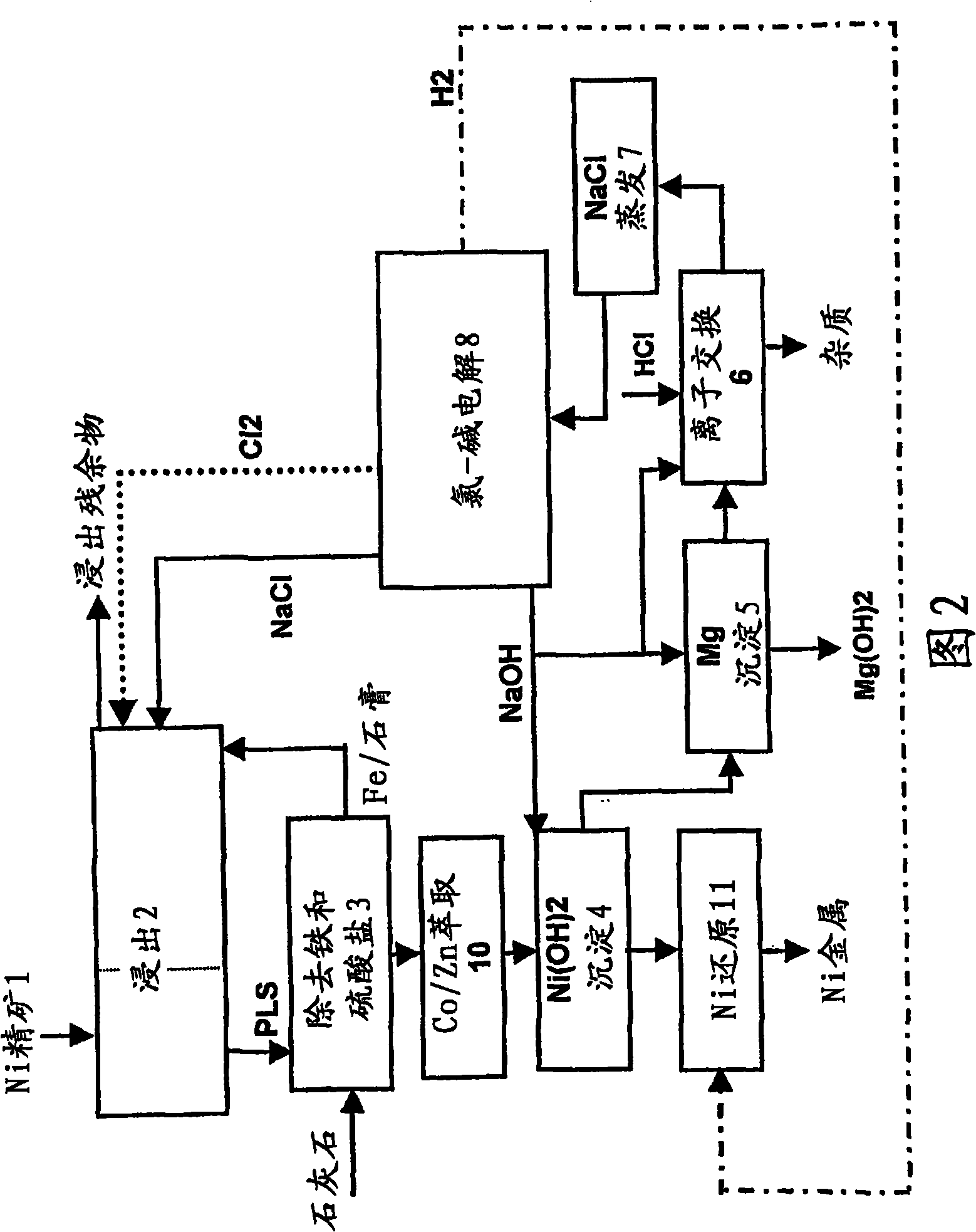
Embodiment 2
[0116] The nickel sulphide concentrate according to Example 1 was leached into a chloride solution similar to Example 1. The first leaching step was carried out in a similar manner to Example 1, but in the second leaching step oxidation of sulfide and cupric chloride was carried out directly by chlorine gas.
[0117] The test was carried out by oxidation of the leach residue from the first step and the feed solution of the second step. The analysis of the product solution and precipitate is given below:
[0118] the solution
[0119]
[0120] solid (leach residue)
[0121]
[0122] The solution from the second leaching step was subjected to the first leaching step, after which the solution was further processed in a similar manner to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com