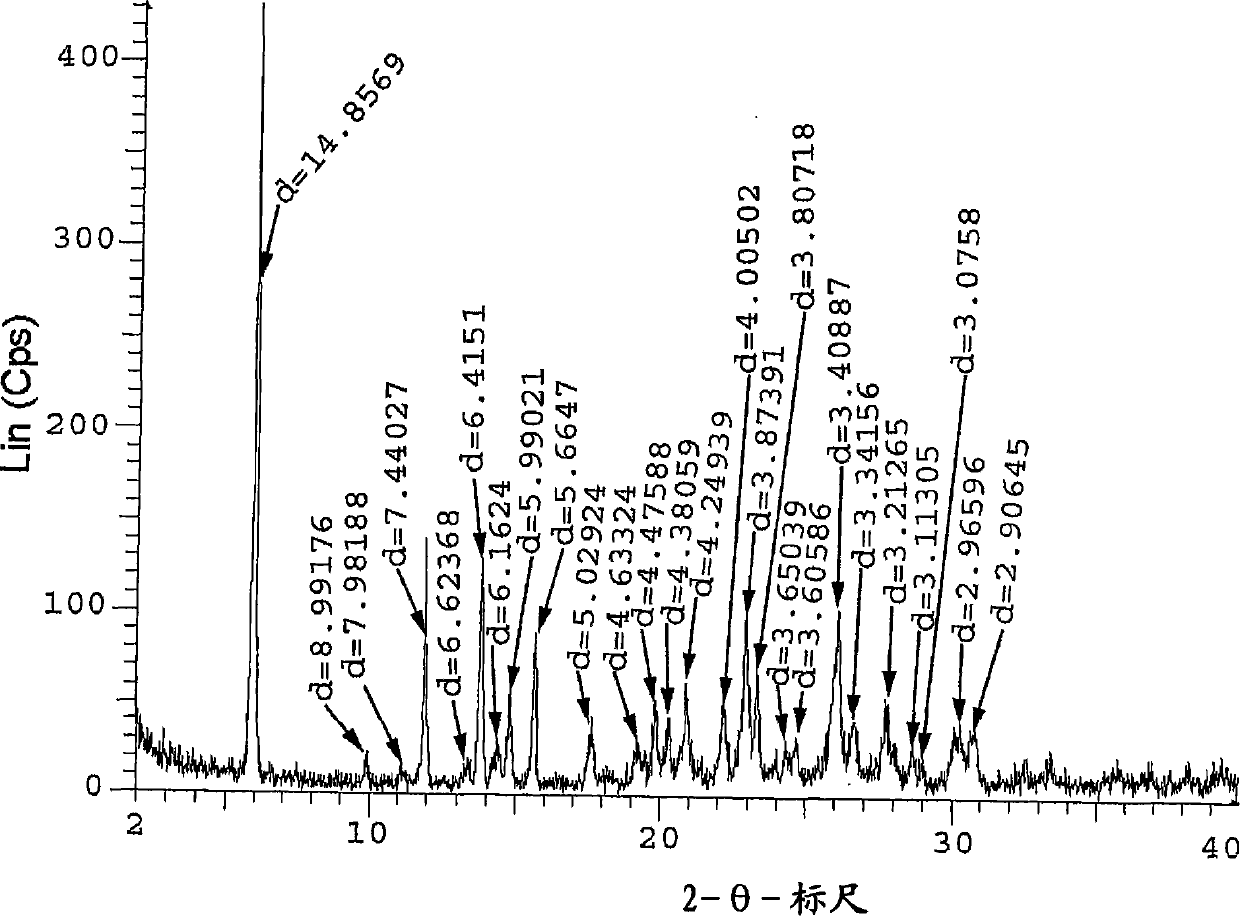
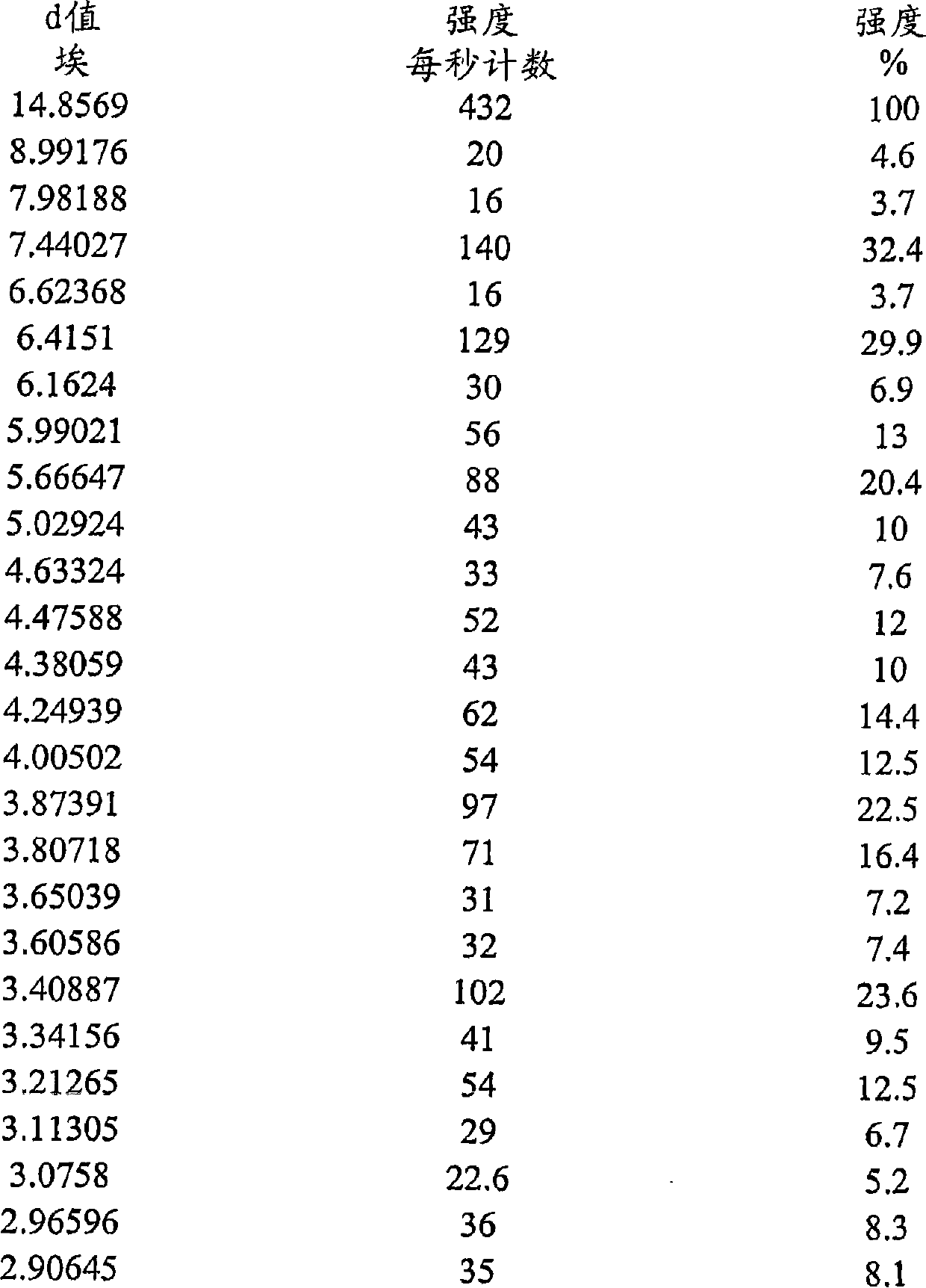
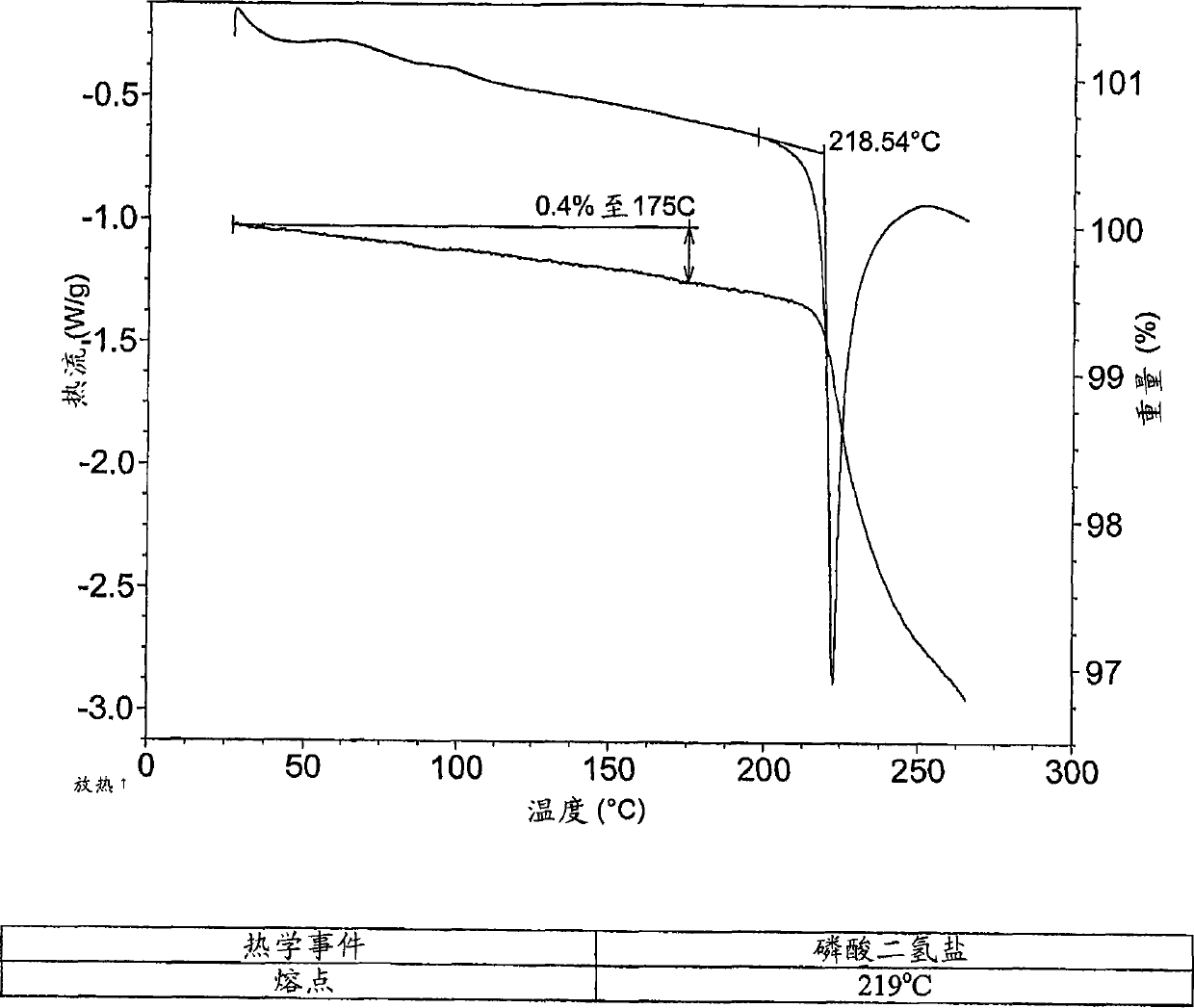
Dihydrogen phosphate salt of a prostaglandin d2 receptor antagonist
A pharmaceutical carrier and drug technology, which is applied in the field of dihydrogen phosphate, can solve problems that are not specifically disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0047] A particular embodiment of the invention is the compound of formula (III) in crystalline form.
[0048] The compounds of the present invention exhibit prostaglandin D2 receptor antagonist activity and are useful pharmacologically active agents. Therefore, it is incorporated into pharmaceutical compositions and used to treat patients suffering from certain medical disorders.
[0049]Compounds of the present invention are antagonists of the prostaglandin D2 receptor according to assays described in the literature and in the Pharmacological Assays section below, the results of which are believed to correlate with pharmacological activity in humans and other mammals. Accordingly, in another embodiment, the present invention provides compounds of the invention and pharmaceutical compositions containing the compounds of the invention for use in the treatment of patients suffering from or susceptible to a condition ameliorated by administration of a PGD2 antagonist. patient. ...
Embodiment
[0117] 2-(3-{6-[2-(2,4-Dichlorophenyl)ethylamino]-2-methoxypyrimidin-4-yl}phenyl)-2-methylpropanoic acid dihydrogen phosphate
[0118] Step 1: 4,6-dichloro-2-methoxypyrimidine (0.7g), 2,4-dichlorophenethylamine (0.82g) and sodium bicarbonate (0.88g) were dissolved in ethanol (25mL) The solution was heated at 80°C for 3 hours and poured into water (400 mL). The resulting solid was filtered and air dried to give (6-chloro-2-methoxypyrimidin-4-yl)-[2-(2,4-dichloro Phenyl)ethyl]-amine .
[0119] Step 2: To a solution of lithium diisopropylamide in tetrahydrofuran / n-heptane / ethylbenzene (1.8 M, 17 mL) at 0 °C was added dropwise 2-(3-bromo-phenyl)- A solution of propionic acid (3 g, 13.9 mmol) in tetrahydrofuran (5 mL). The mixture was stirred for 1 hour, then a solution of methyl iodide (4.93 g, 34.8 mmol) in tetrahydrofuran (5 mL) was added dropwise over 10 minutes. The reaction mixture was stirred for 15 hours, quenched with 2N HCl, concentrated in vacuo, and diluted w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com