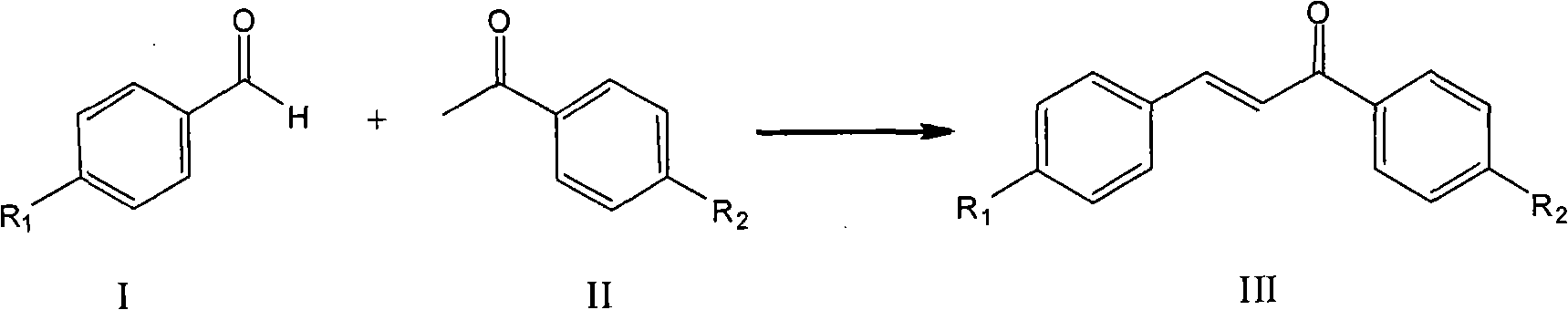
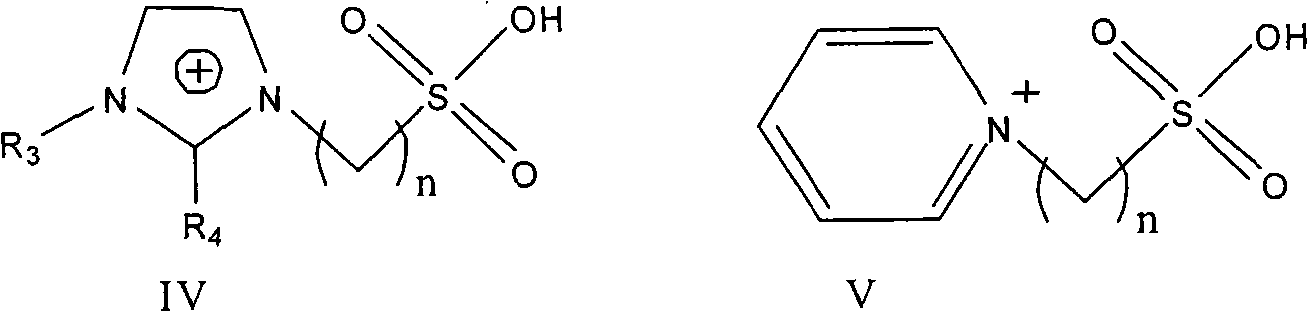
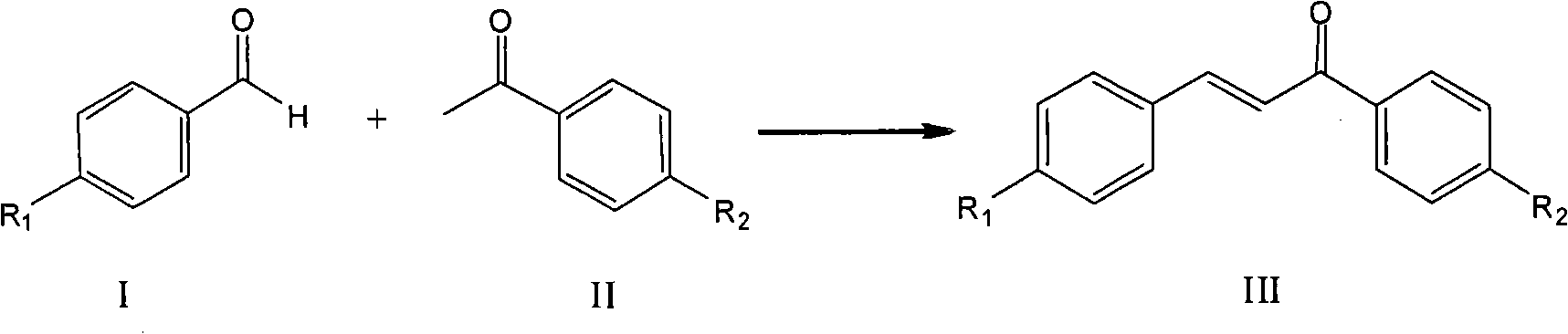
Process for synthesizing chalcone and derivates thereof by using ion liquid
An ionic liquid and chalcone technology, which is applied in the synthesis field of producing chalcone and its derivatives, can solve the problems of complicated separation and purification, and achieve the effect of easy separation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 3.58g (0.01mol) ionic liquid 3-butyl-1-(butyl-4-sulfonic acid) imidazolium bisulfate, 6.01g (0.05mol) acetophenone, 5.31g (0.05mol) benzaldehyde In a three-necked flask equipped with a stirrer, a thermometer, and a reflux condenser, stir and raise the temperature to 140°C, and react for 6 hours. After the reaction solution is allowed to stand and separate, the chalcone product in the upper layer is poured out, recrystallized and purified, 1,3- The yield of diphenyl-2-propen-1-one (chalcone) is 96.9%, and the selectivity is 99.8%. The lower ionic liquid was dried in vacuum at 110 °C for 2 h and reused.
Embodiment 2-10
[0037] Embodiment 2-10: the specific experimental process is similar to embodiment 1
[0038] Reality
Embodiment 11-13
[0040] 6.01g (0.05mol) acetophenone, 5.31g (0.05mol) benzaldehyde, reaction temperature 140°C
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com