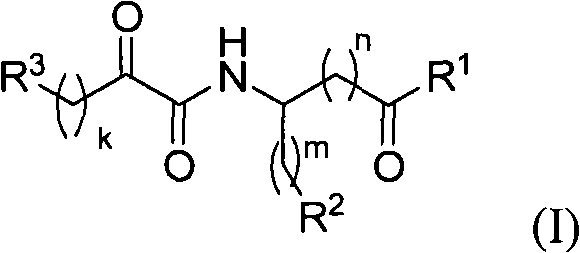
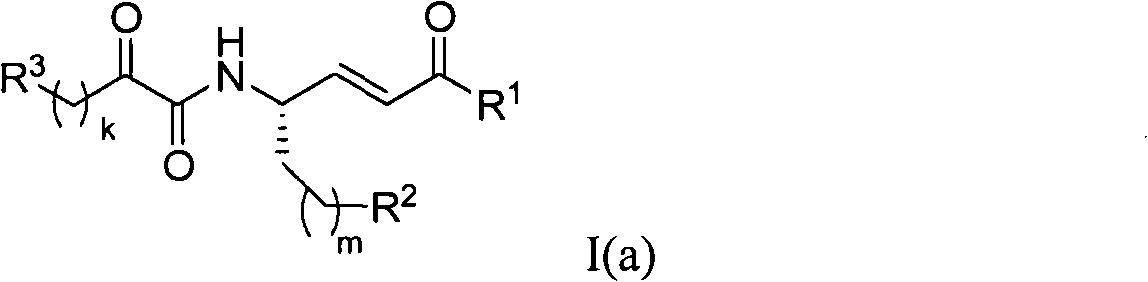
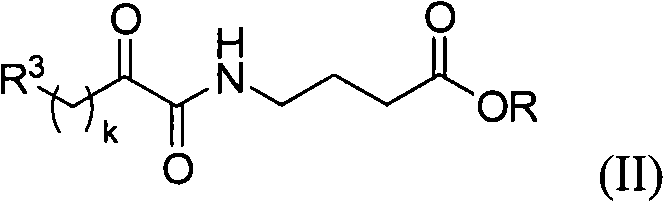
Systemic and intrathecal effects of a novel series of phospholipase a2 inhibitors on hyperalgesia and spinal pge2 release
A cell-inhibiting, unsaturated technology, applied in the direction of anti-inflammatory agents, nervous system diseases, active ingredients of ketones, etc., can solve problems such as difficult and difficult to design selective inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
Hyperalgesia Animal Model and Measurement Method
animal
[0085] Male Holtzman Sprague-Dawley rats (300-350 g; Harlan Industries) were housed individually and maintained on a 12-hour light / dark cycle with free access to food and water.
Intrathecal Catheter Implantation
[0086] For spinal drug injection, a lumbar catheter was implanted in the rat under isoflurane anesthesia according to a modification of the procedure described by Yaksh (Yakshand Rudy, supra, 1976). A polyethylene catheter (PE-5; Spectranetics, OD 0.014) was inserted into the intrathecal space and advanced through an incision in the atlanto-occipital membrane to the rostral border of the lumbar enlargement. Rats entered the study five days after implantation. In a separate experiment evaluating spinal cord prostaglandin release, rats were prepared with a lumbar loop dialysis catheter with three lumens, as described previously, see (Yaksh, et al., supra, 2001).
[0087] Briefly, the outer two lumens were conn...
Embodiment II
Treatment of carrageenan-induced thermal hyperalgesia following intraperitoneal delivery
[0098] Control. Baseline thermal escape latency was at the level of 10-12 seconds in all groups prior to induction of hyperalgesia. Plantar injections of carrageenan induced inflammation in the injected hindpaw and corresponding thermal hyperalgesia detectable after a duration of 60 min throughout the study. As shown in Figure 5, the heat escape latency in animals treated with IP or IT vehicle was significantly reduced to about 3-5 seconds within 90-120 minutes (see Figures 5 and 6).
[0099] Intraperitoneal delivery. Pretreatment (30 min) with four substances at 3 mg / kg (IP) before carrageenan injection revealed that AX048, but not AX006, AX010 or AX057, lowered the rate of inflamed paw otherwise. Thermal hyperalgesia was observed (Figure 5). Importantly, thermal escape latencies in the healthy paw were not altered in vehicle- or drug-treated animals, eg, the substance functions as an an...
Embodiment III
Treatment of carrageenan-induced thermal hyperalgesia following intrathecal delivery
[0102] Control. In animals receiving intrathecal injections of vehicle, plantar injections of carrageenan resulted in marked unilateral thermal hyperalgesia compared to non-injected paws (FIG. 8).
[0103] Drug Effects Pretreatment with 30 μg / 10 μL of the four substances revealed that AX048, but not AX006, AX010 or AX057, reduced thermal hyperalgesia 15 minutes before carrageenan delivery (see Figure 8). Furthermore, following intrathecal delivery, there was no change in heat escape latency in the healthy paw in either the vehicle or drug-treated groups. Comparison of mean group differences between response latencies in healthy and injured paws also revealed a significant reduction in the AX048-treated group compared to the vehicle-treated group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com