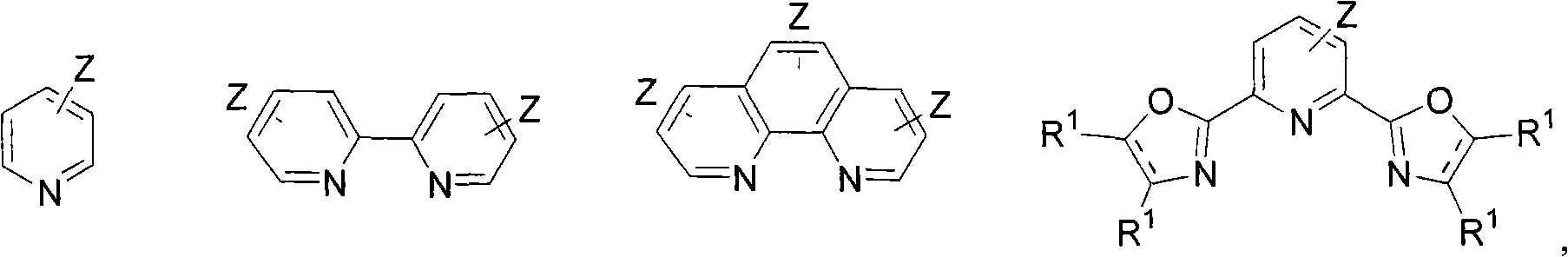
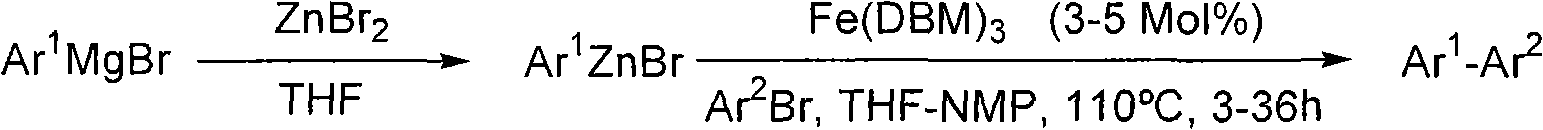Nickel or iron catalysed carbon-carbon coupling reaction of arylenes, alkenes and alkines
A catalyst and aryl technology, applied in the field of carbon-carbon bonds, can solve the problems of dehalogenation of aryl halides, no synthesis method found, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0059] Unless otherwise indicated, all reactions were performed by magnetic stirring and in the case of air-sensitive or hygroscopic compounds under argon as the inert gas in annealed glassware. Syringes were used to transfer reagents and solvents were flushed with argon before their use. Reactions were controlled by gas chromatography (GC and GC-MS) or thin layer chromatography. If not stated otherwise, solutions of organomagnesium compounds were prepared by reacting magnesium with aryl bromides in THF and treated with I 2 Standard solutions were titrated in 0.5 M LiCl in THF and diluted with THF to the indicated concentrations. ZnBr 2 and ZnCl 2 Dry at 140° C. under high vacuum for 30 min and then dissolve in anhydrous THF.
[0060] General Rule 1 (AV1): Nickel-catalyzed chemical reactions
[0061] A solution of the nickel catalyst was prepared as follows: anhydrous nickel chloride (8.2 mg, 0.063 mmol), (EtO) 2 P(O)H (34.5 mg, 0.25 mmol) and DMAP (30.5 mg, 0.25 mmol) w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com