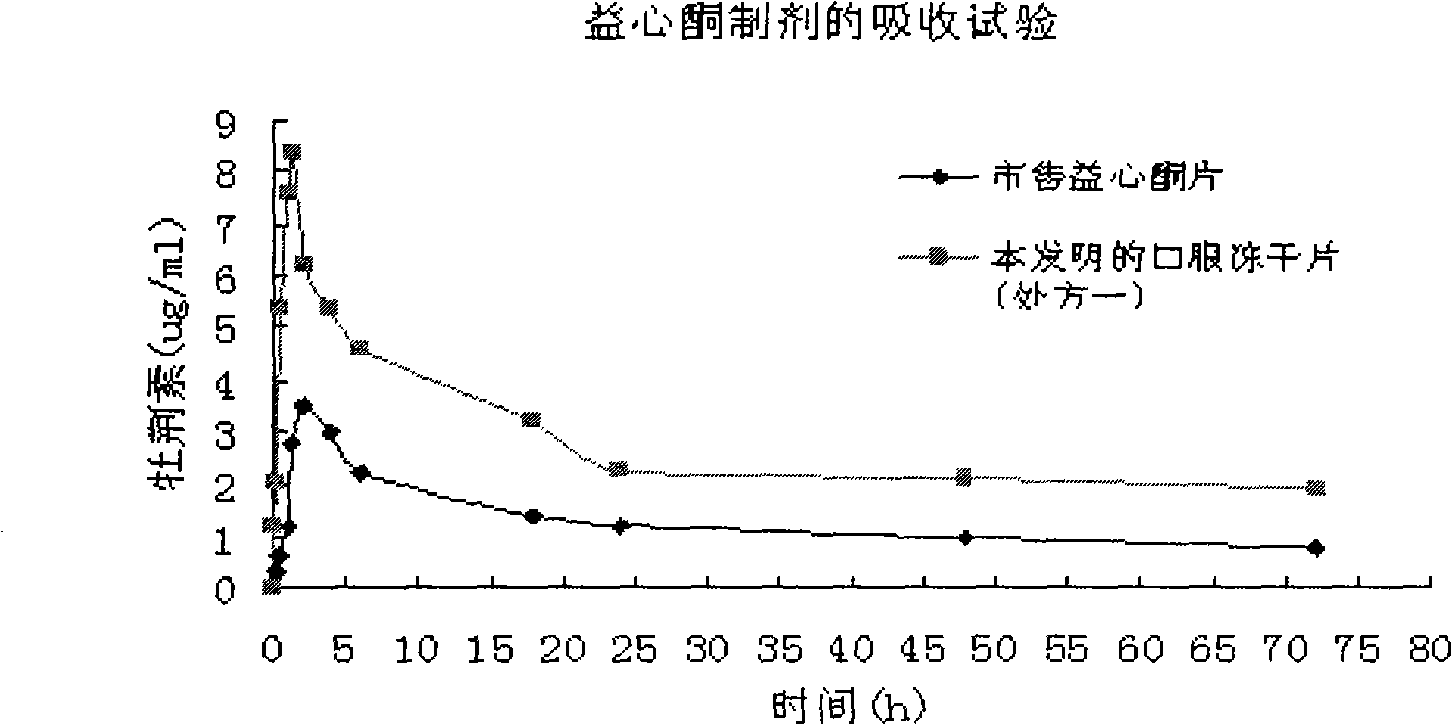
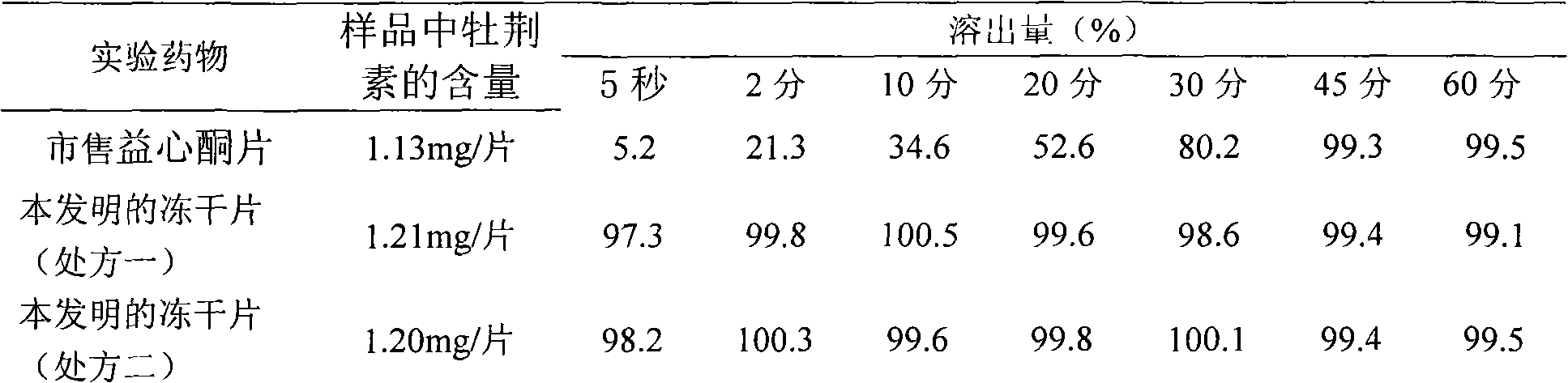
Crataegutt oral freeze-dried tablet and preparation thereof
A technology for freeze-dried tablets and Yixin ketone is applied in the field of traditional Chinese medicine preparations and can solve the problems of low bioavailability, slow dissolution of dosage forms, and difficulty in swallowing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 The preparation of oral freeze-dried tablet of the present invention
[0028]
[0029]
[0030] Preparation:
[0031] Step 1: Take the above-mentioned excipients and place them in a 2000ml container, add an appropriate amount of water, stir and dissolve at a temperature of 50°C to 70°C, and form a colorless and clear solution.
[0032] Step 2: Weigh the total flavonoids of hawthorn leaf as the main drug in the prescribed amount, place it in the above solution, and stir to dissolve it into a dark red solution.
[0033] Step 3: Add water to the full amount and stir to mix evenly.
[0034] Step 4: Take the above-mentioned mixed solution and pack it into molds by 4.5ml, and then make the finished product by the following freeze-drying process.
[0035] Freeze-drying process: Pre-freeze at -60°C for 3 to 5 hours. Under vacuum and low temperature conditions, the sample is dried at a rate of 4°C / h to 0°C for the first drying, and then at a rate of 6°C / h to...
Embodiment 2
[0041] Embodiment 2 Preparation of oral freeze-dried tablet of the present invention
[0042]
[0043] Preparation:
[0044] Step 1: Take the above-mentioned excipients and place them in a 2000ml container, add an appropriate amount of water, stir and dissolve at a temperature of 50°C to 70°C, and form a colorless and clear solution.
[0045] Step 2: Weigh the total flavonoids of hawthorn leaf as the main drug in the prescribed amount, place it in the above solution, and stir to dissolve it into a dark red solution.
[0046] Step 3: Add water to the full amount and stir to mix evenly.
[0047] Step 4: Take the above-mentioned mixed solution and pack it into molds by 4.5ml, and then make the finished product by the following freeze-drying process.
[0048]Freeze-drying process: pre-freezing at 60°C for 3-5 hours, under vacuum and low temperature conditions, the sample is dried at a rate of 4°C / h to 2°C for the first drying, and then at a rate of 6°C / h to 30 ℃ for secondar...
Embodiment 3
[0054] Embodiment 3 The preparation of oral freeze-dried tablet of the present invention
[0055]
[0056] Preparation:
[0057] Step 1: Take the above-mentioned excipients and place them in a 2000ml container, add an appropriate amount of water, stir and dissolve at a temperature of 50°C to 70°C, and form a colorless and clear solution.
[0058] Step 2: Weigh the total flavonoids of hawthorn leaf as the main drug in the prescribed amount, place it in the above solution, and stir to dissolve it into a dark red solution.
[0059] Step 3: Add water to the full amount and stir to mix evenly.
[0060] Step 4: Take the above-mentioned mixed solution and pack it into molds by 4.5ml, and then make the finished product by the following freeze-drying process.
[0061] Freeze-drying process: pre-freeze at -60°C for 3 to 5 hours, and then rise to -2°C at a rate of 4°C / h for the first drying of the sample under vacuum and low temperature conditions, and then rise to Perform secondar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com