Tripterine derivate, preparation method and use thereof
A technology of tripterycin and medicinal salts, which is applied in the field of tripteryne derivatives and their preparation and application, and can solve the problems of high toxicity and poor water solubility of tripteryne
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Preparation of tripterine cetyl alcohol ester
[0029] In a dry round bottom flask, add 32g tripterine, 14g DCC and 600mL CH 2 Cl 2 , add 19 g of n-hexadecanol dropwise in CH with stirring 2 Cl 2 (400mL) solution, reacted at room temperature for 24h. After the reaction was completed, 300 mL of H was added to the reaction solution. 2 O, with CH 2 Cl 2 Extraction, the organic phase was concentrated and evaporated to dryness. The product was separated by silica gel column chromatography to obtain 31 g of tripterine cetyl alcohol ester as a red solid with a yield of 65%.
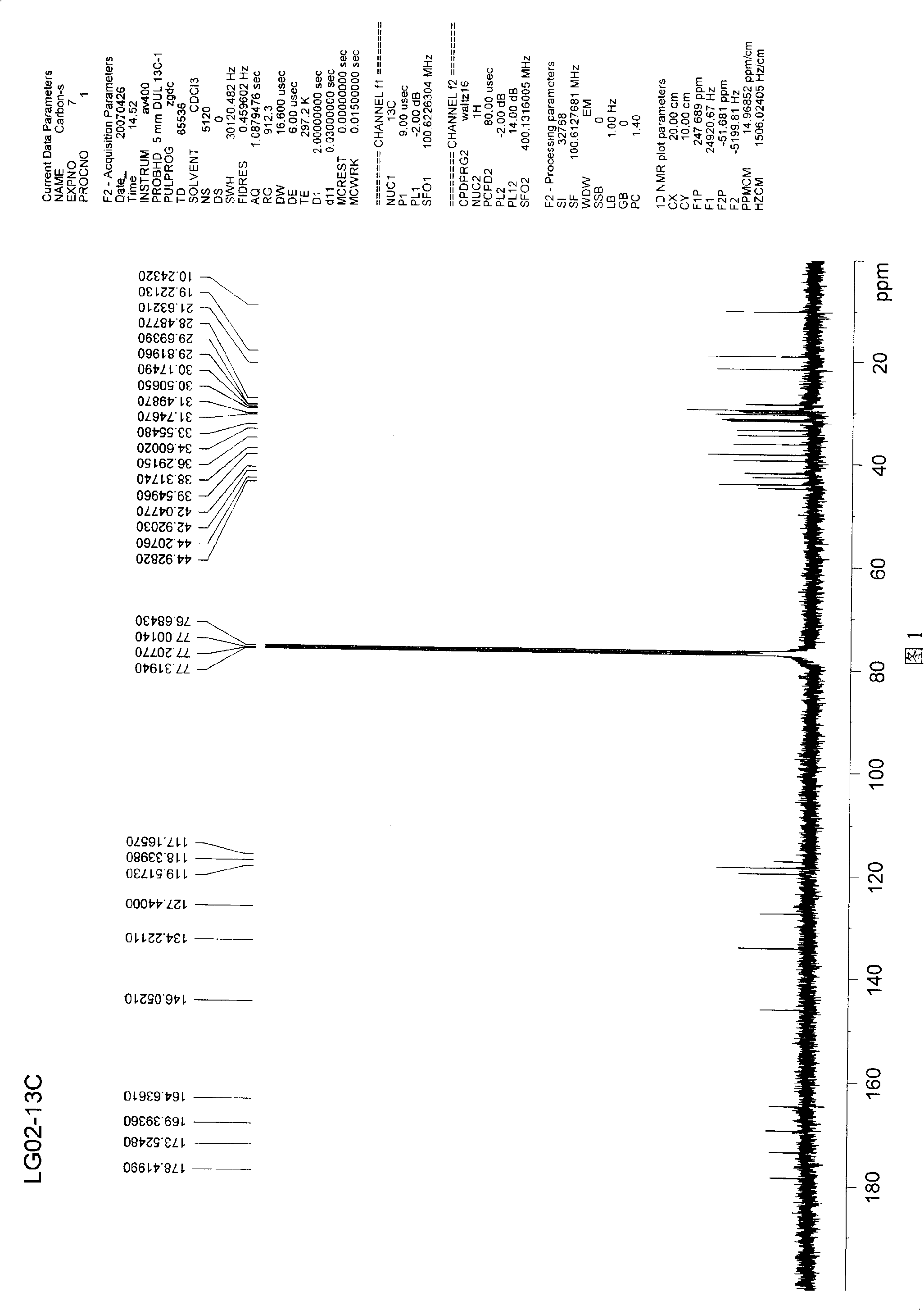
[0030] The confirmed data of the chemical structure of tripterygium cetyl alcohol ester are as follows:
[0031] Molecular formula: C 45 h 70 o 4 (674)
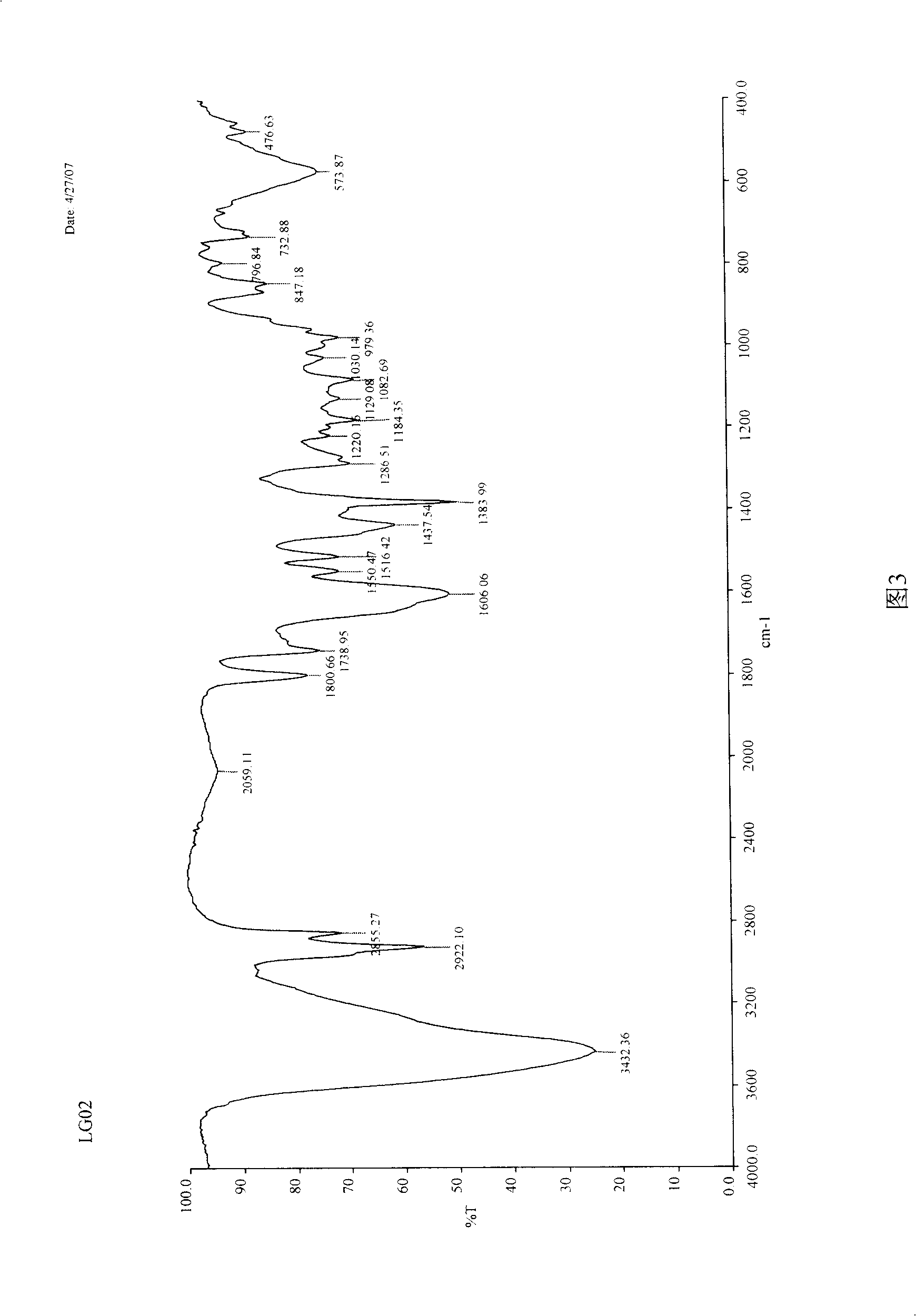
[0032] IR v max KBr (cm -1 ): 3432, 2922, 2855, 1800, 1739, 1606, 1550, 1516, 1437, 1383, 1286, 1184, 1129, 1082, 979, 847, 732, 573
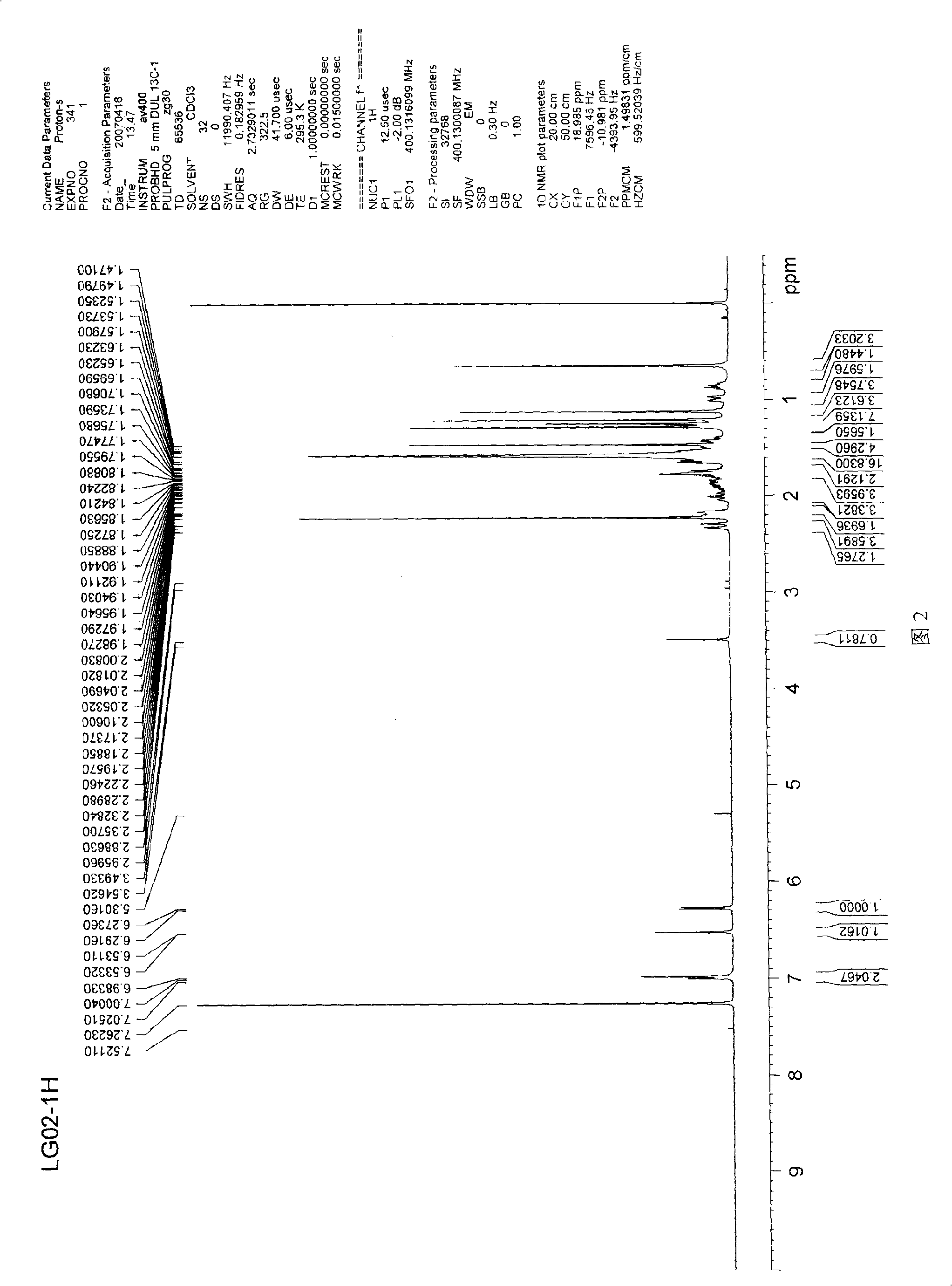
[0033] 1 H-NMR (400MHz, CDCl 3 ): 7.03(s, 1H, 3-OH), 6.99(br d, J=7.00Hz, 1H, H-6), 6.5...
Embodiment 2
[0036] Embodiment 2: the preparation of tripterine cetyl alcohol ester
[0037] Add 32g tripterine and an appropriate amount of toluene in turn to a dry round bottom flask, add dropwise a toluene solution of 9.5g n-hexadecanol under stirring, and react at 110°C for 30 minutes. After the reaction was completed, an appropriate amount of H was added to the reaction solution. 2 O, with CH 2 Cl 2 Extraction, the organic phase was concentrated and evaporated to dryness. The product was separated by silica gel column chromatography to obtain 20.6 g of tripterine cetyl alcohol ester as a red solid with a yield of 43%.
Embodiment 3
[0038] Embodiment 3: the preparation of tripterine cetyl alcohol ester
[0039] Add 32g of tripterine, 10g of DCC and appropriate amount of carbon tetrachloride successively in a dry round bottom flask, add 12.6g of n-hexadecanol solution of carbon tetrachloride dropwise under stirring, and react at 0°C for 48h. After the reaction was completed, an appropriate amount of H was added to the reaction solution.2 O, with CH 2 Cl 2 Extraction, the organic phase was concentrated and evaporated to dryness. The product was separated by silica gel column chromatography to obtain 27.8 g of tripterine cetyl alcohol ester as a red solid with a yield of 58%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com