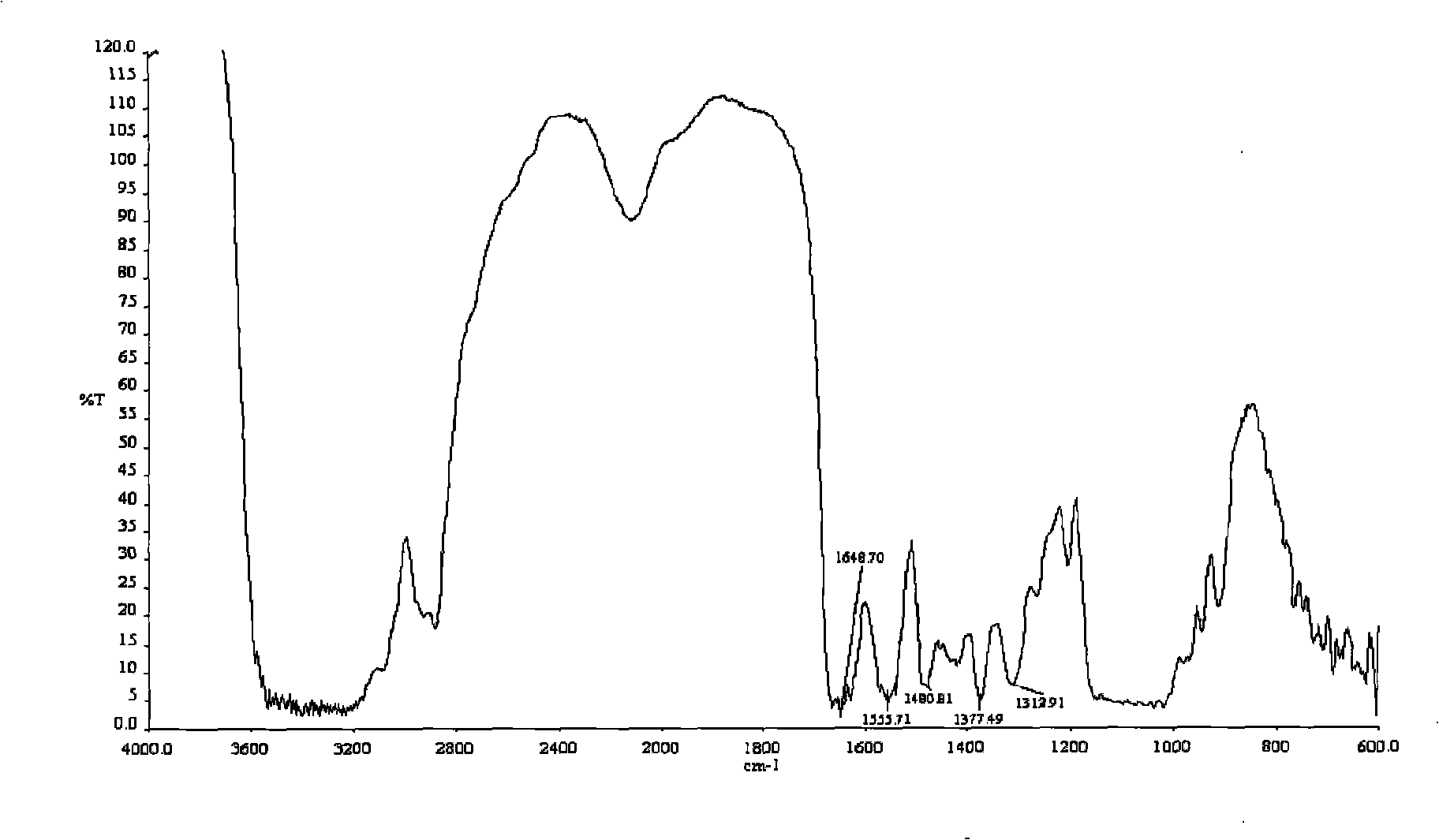
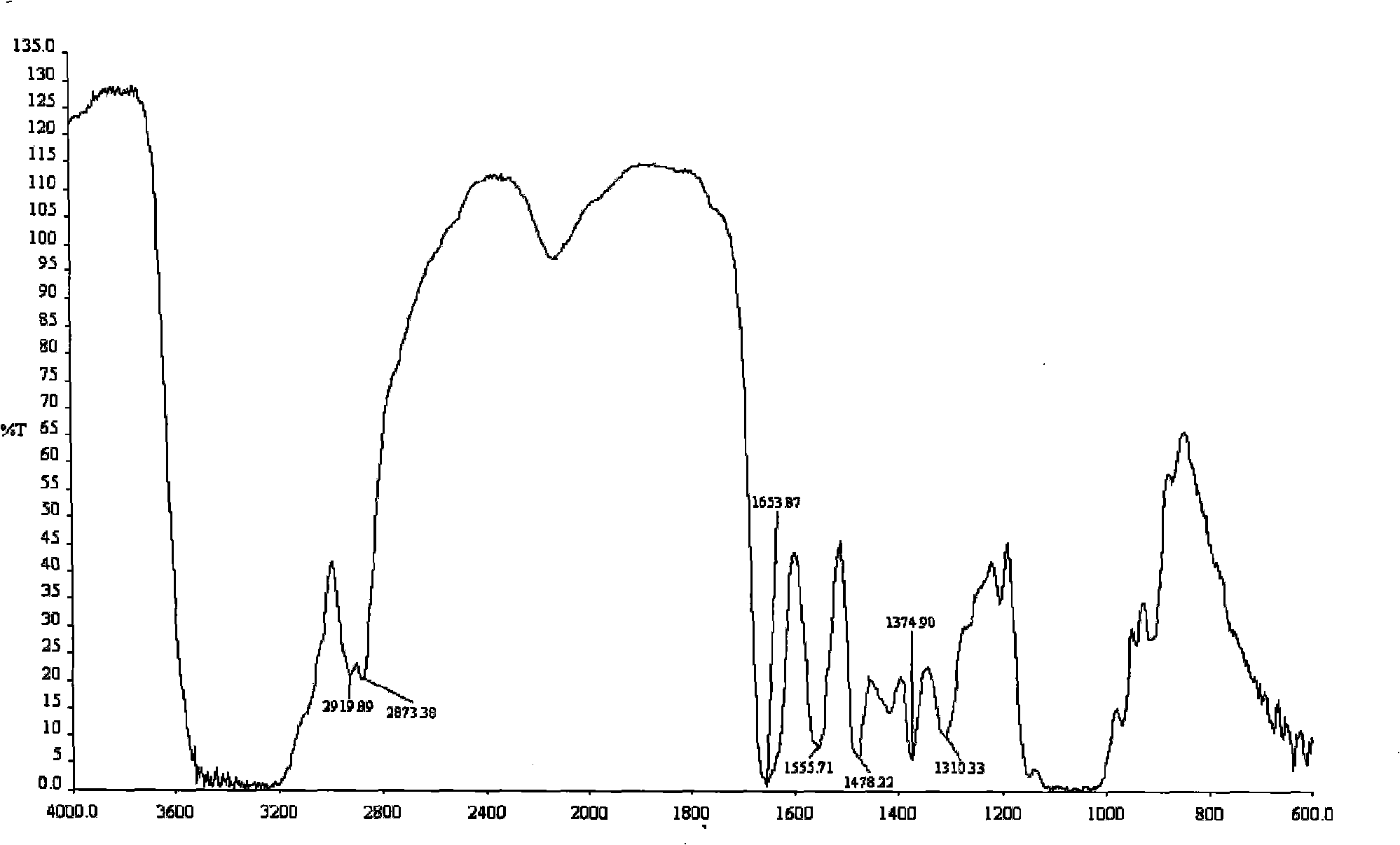
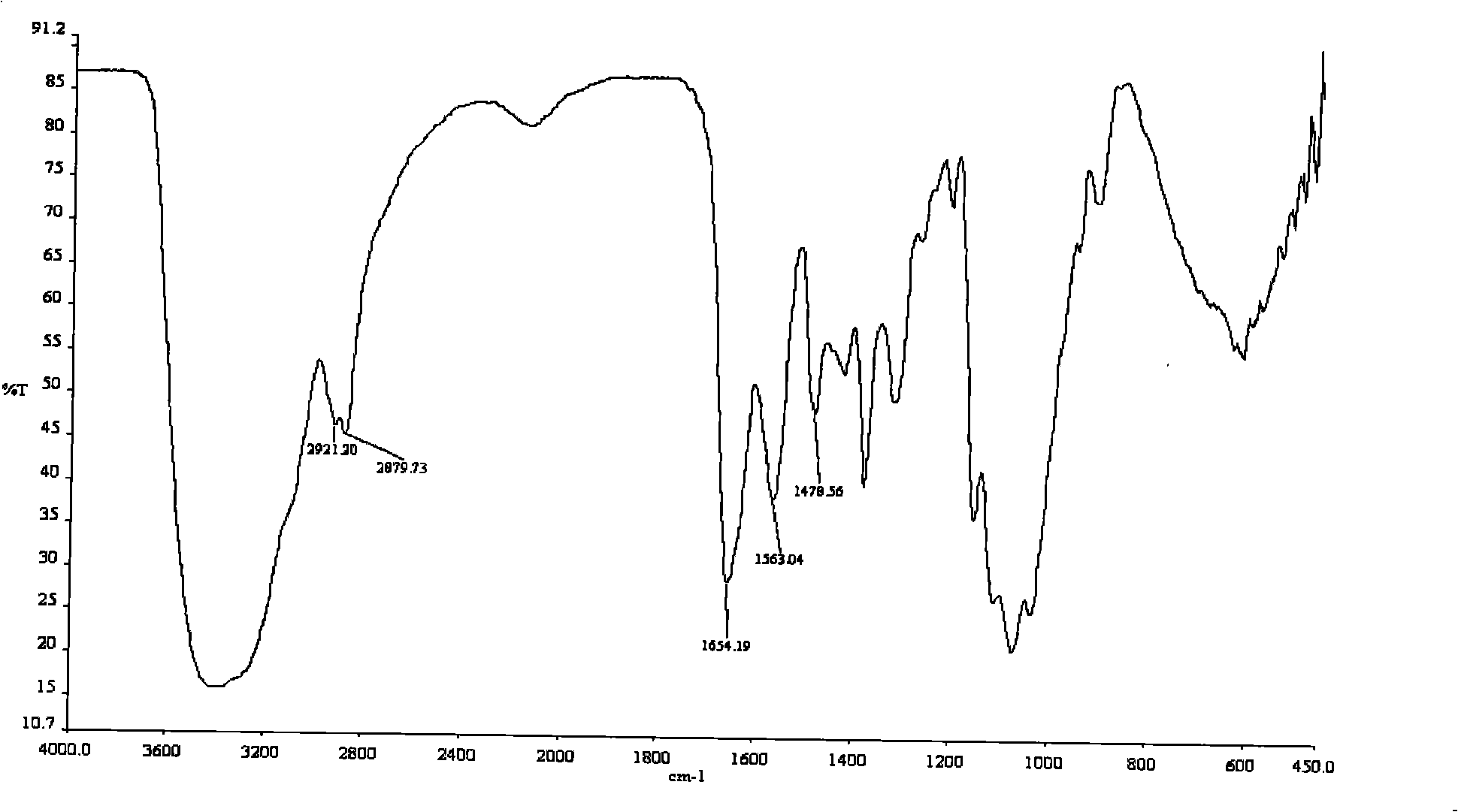
Process for preparing O-2'-hydroxypropyl trimethyl ammonium chloride chitosan
The technology of hydroxypropyltrimethylammonium chloride and glycidyltrimethylammonium chloride is applied in the field of quaternary ammonium salting of chitosan, can solve the problems of complex synthesis method and the like, achieves high reactivity, The effect of reducing the degree of degradation reaction and easy adjustment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0030] Examples 1-9 prepared O-2'-HTACCt according to the above-mentioned step a, and prepared O-2'-HTACCts according to the above-mentioned step b. In Examples 1-9, different β-chitin particle sizes, modifiers, modifier / β-chitin molar ratios, substitution reaction temperatures, substitution reaction times, and dispersion solvents were used. The conditions for preparing O-2'-HTACCt and the degree of substitution of the product are shown in Table 1. The molar ratio in Table 1 refers to the modifier / β-chitin molar ratio. A in the modifier is 2,3-GTAC, and B is 3-Cl-2-HTAC.
[0031] Table 1 Relevant parameters of the substitution reaction
[0032] Example
Particle size μm
modifier
Dispersion solvent
temperature °C
The molar ratio of
time h
1
5
A
Isopropanol
20
0.5∶1
12
33.30
2
10
B
Isopropanol
40
4∶1
6
71.06
3
300
A+B
...
Embodiment 9
[0054] In Example 9, O-2'-HTACCt was prepared by deacetylation, and the degree of deacetylation of the O-2'-HTACCts sample was 65.32%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com