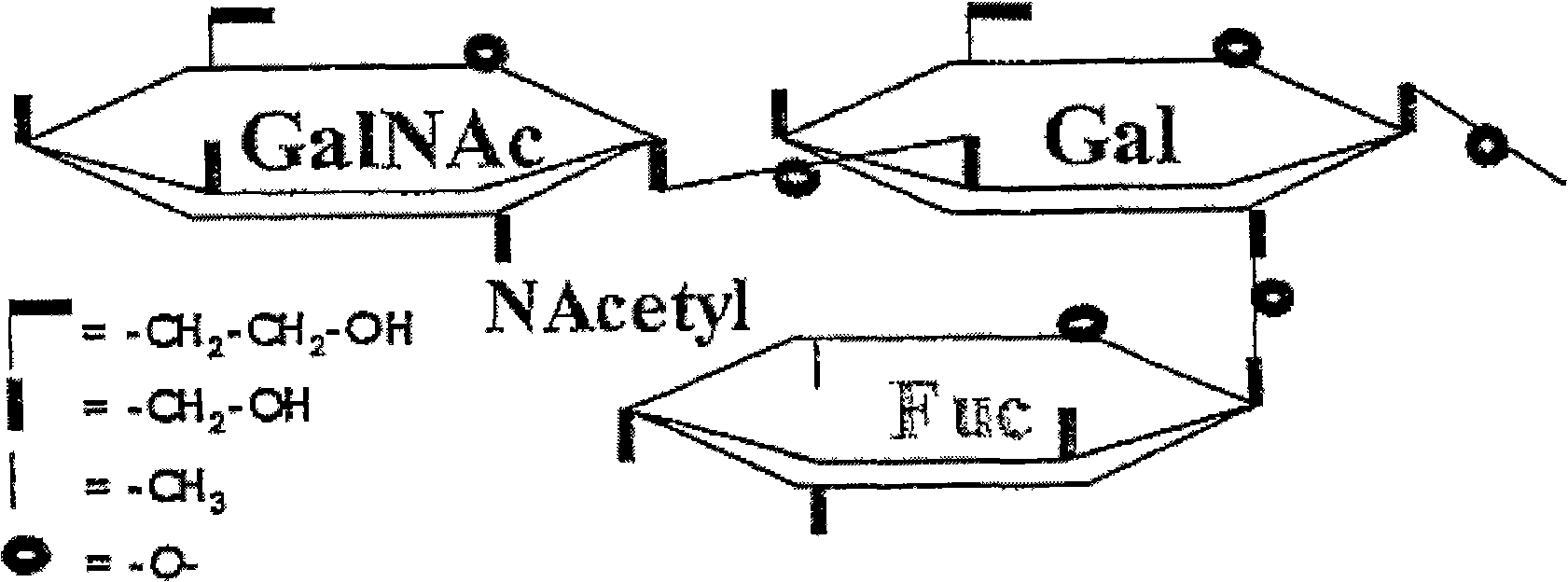
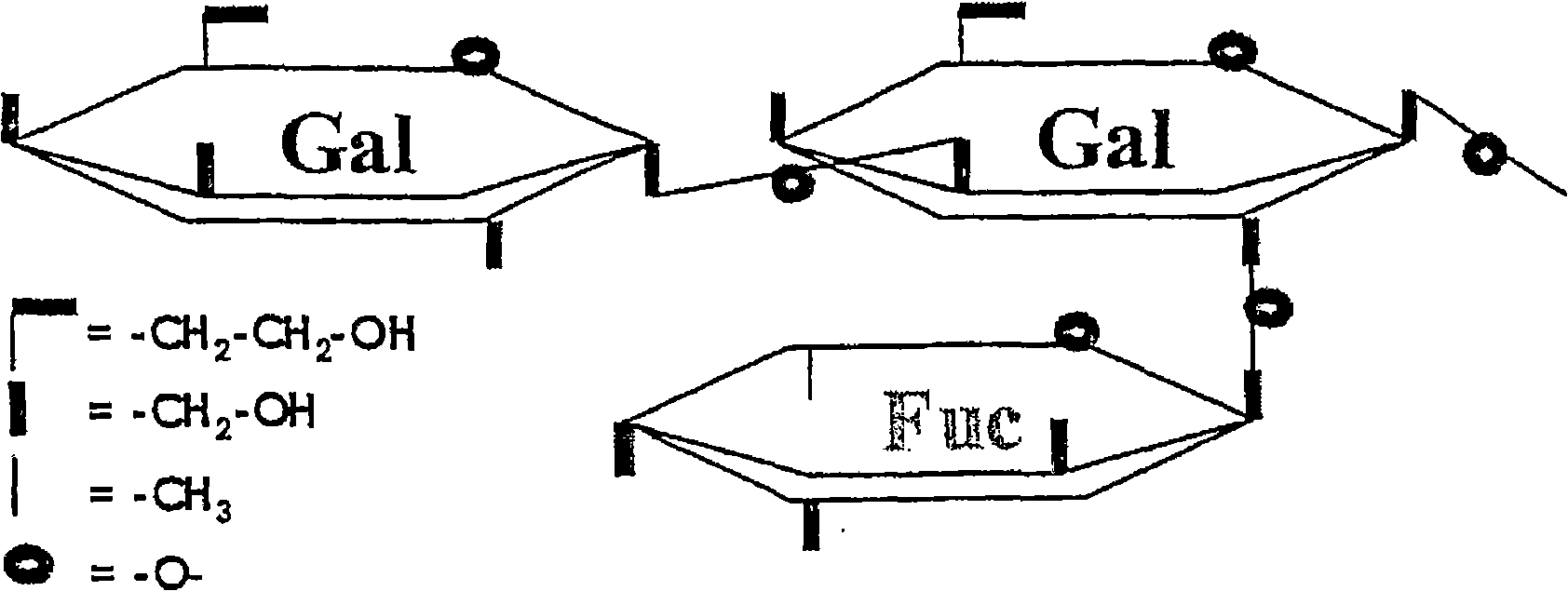
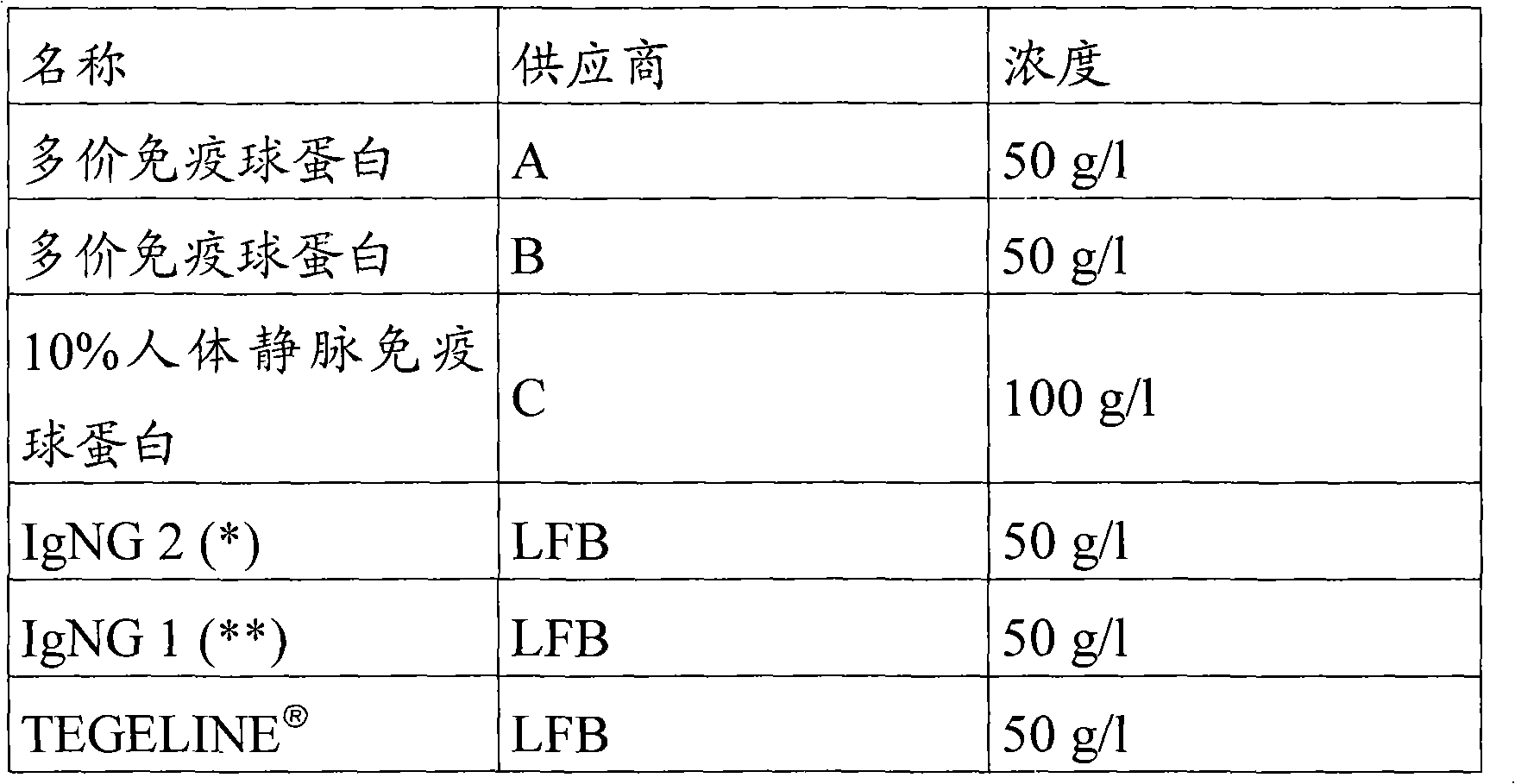
Immunoglobulin G (IGG) concentrate depleted of anti-A and anti-B antibodies and of polyreactive IGGS
A technology of immunoglobulin, polyreactivity, applied in the field of obtaining the above-mentioned concentrate, capable of solving problems such as adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] A sample of IgG concentrate (B2) at 40 g / l was obtained following the method described in WO 02 / 092632.
[0133] A chromatographic column with a length of 50 cm and a diameter of 44 mm was loaded with a 50 / 50 (v / v) GLYCOSORB ABO medium mixture, which was grafted with trisaccharides equivalent to blood type A and blood type B epitopes, and then pre- Rinse it with 1200ml of water.
[0134] Inject the B2IgG concentrate at a rate of 0.21 / ml medium with the pump. Once the contents have permeated the cylinder, the cylinder is flushed with a minimum volume of injectable ready water (IP) to collect the IgG present in the column dead volume.
[0135] Antibody A and Antibody B and polyreactive IgG were removed from which a B3IgG concentrate of about 40 g / l was collected, then ultrafiltration was used to obtain a concentrate of 60 g / l, viruses were removed by nanofiltration on three series of filters, and Decreasing limits of persistence are obtained at 100, 50 and 20nm.
[013...
Embodiment 2
[0137] Example 2: Quantitative analysis of anti-A / Bs in IgIVs
[0138] 1) Test principle
[0139] 1-1) Preparation of human red blood cells
[0140] Normalize human erythrocyte suspensions of A Rhesus+, B Rhesus+, or O Rhesus+ blood type to 40 × 10 6 Concentration of erythrocytes / ml in PBS buffer at pH=7.4+1% BSA.
[0141] 1-2) Prepare the monoclonal D antibody range
[0142] A monoclonal D antibody (designated R297) was prepared for detection of absorbance (OD) at 280 nm relative to PBS buffer at pH 7.4. Relative to the composition in different amino acids, the molar extinction coefficient (ε) of the protein was calculated, and the concentration of monoclonal anti-D was obtained by the following formula:
[0143] C=OD / E1, where 1 is equal to the width of the container used for OD determination.
[0144] A monoclonal anti-D antibody ranging from 0 to 200 ng / ml was produced at 12 points (200; 150; 100; 75; 50; 25; 12.5; 6.25; 3.13; 1.56; 0.78 and Ong / ml).
[0145] 1-3) ...
Embodiment 3
[0175] A 1% (v / v) suspension of erythrocytes in blood group A was prepared in PBS buffer, pH 7.4, containing 1 wt.% bovine serum albumin (BSA). 50 μl of erythrocyte suspension was taken out and added to a flow cytometry tube (Beckmann-Coulter Epics XL)) and 50 μl of internal standard solution to measure the flow rate. The suspension is calibrated to 40.10 6 RBC / ml.
[0176] Eight IgG solutions were prepared by serially diluting the IgG concentrate (v / v) (B3) obtained from Example 1 by a factor of 2, with a maximum concentration of 30 g / l and a maximum dilution of 0.234 g / l . Then, a volume of 50 μl of the suspension was put into each well of a 96-well microplate, and then 50 μl of IgG solutions of different dilutions were added.
[0177] The whole solution was incubated at 37°C for 2 hours with stirring.
[0178] Each well was washed with 200 μl of PBS buffer including the aforementioned BSA, and the microplate was centrifuged at 2000 rpm for 1 minute. After clearing the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com