Novel polyurea based on diisocyanates and diacetyl m-phenylenediamine
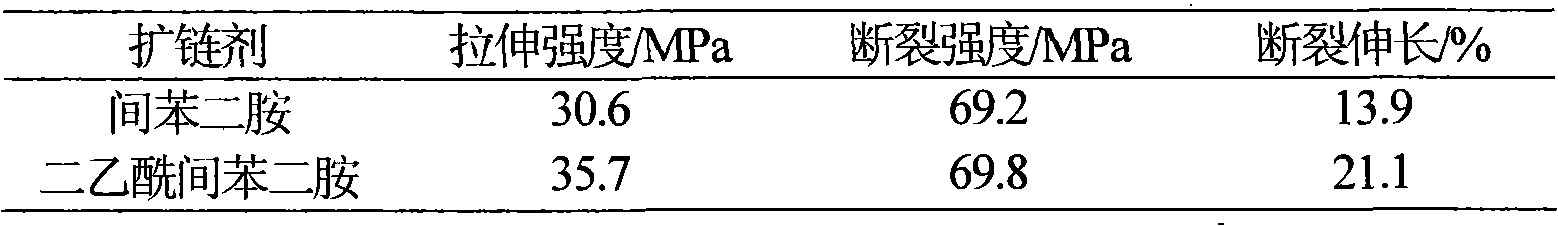
A technology of diacetyl m-phenylenediamine and diisocyanate is applied in the field of polyurea, which can solve the problems of difficult molding of polyurea, high reactivity, difficult to obtain a smooth coating surface effect, etc. Effects of gel time and reactivity reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
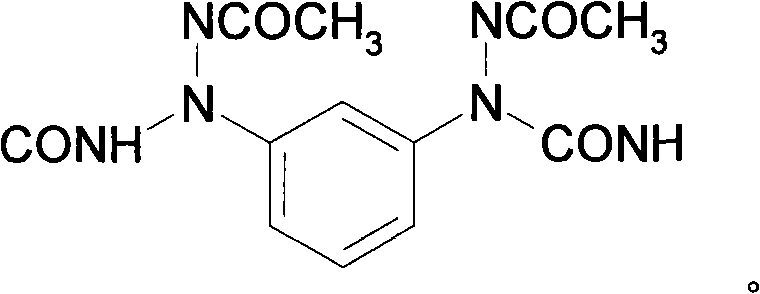
[0017] Dissolve diphenylmethane diisocyanate MDI in the solvent N,N-dimethylformamide DMF (ω=0.4), then add a metered amount of chain extender in DMF solution (ω=0.25), and stir evenly at room temperature The amount of MDI used is 200g, and the molar ratio is MDI:chain extender=1.05:0.61; then evaporate the solvent at 60°C and continue the reaction for 30 hours, and finally evaporate the solvent at 80°C for 1 hour and 110°C for 3 hours And continue to react to constant weight. Infrared detection concluded that the new chain extender reacted with isocyanate to synthesize polyurea. A polyurea chain-extended with diacetyl m-phenylenediamine is obtained, the structure of which is shown above. All products are white / colorless elastic solids, insoluble in standard solvents.
Embodiment 2
[0019] Dissolve MDI in DMF (ω=0.3), then add amino-terminated polyether D2000 dropwise, then add a metered amount of chain extender in DMF solution (ω=0.2), and stir evenly at room temperature. The amount of MDI used is 100g, Molar ratio MDI: D2000: chain extender=1.05: 0.39: 0.61; then evaporate the solvent at 60°C and continue the reaction for 30 hours, and finally evaporate the solvent at 80°C for 1 hour and 110°C for 3 hours and continue to react to constant weight. A polyurea cross-linked with amino-terminated polyether D2000 and chain-extended with m-phenylene diacetyl was obtained, the structure of which is shown above. All products were white / yellow elastic solids, insoluble in standard solvents.
PUM
| Property | Measurement | Unit |
|---|---|---|
| gel time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com