Cancer diagnosing kit containing HLA-G monoclonal antibodies and use thereof
A monoclonal antibody, kit technology, applied in biological testing, material testing products, measuring devices, etc., to achieve the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of HGY monoclonal antibody of the present invention
[0041] According to the method disclosed by Ye Shangmian patent: ZL 2004 10040919.X, the anti-HLA-G monoclonal antibody HGY was prepared using the hybridoma cell line with the preservation number CCTCC NO.C200416 preserved in the China Center for Type Culture Collection. The purification of the natural HLA-G protein, the preparation of the monoclonal antibody and the characteristics of the monoclonal antibody are all consistent with the content disclosed in the above patent.
[0042] Preparation of HGY-2 monoclonal antibody of the present invention
[0043] According to Ye Shangmian's patent: the method disclosed in ZL 2004 10040919.X, the basis of the present invention also adopts the preparation method of HGY monoclonal antibody, using the natural HLA-G protein purified from placental tissue aborted within three months of pregnancy, wherein Containing all HLA-G transcript isomers and glyc...
Embodiment 2
[0053] Example 2 Development of HLA-G enzyme-linked immunoassay kit of the present invention
[0054] 1. Monoclonal antibody:
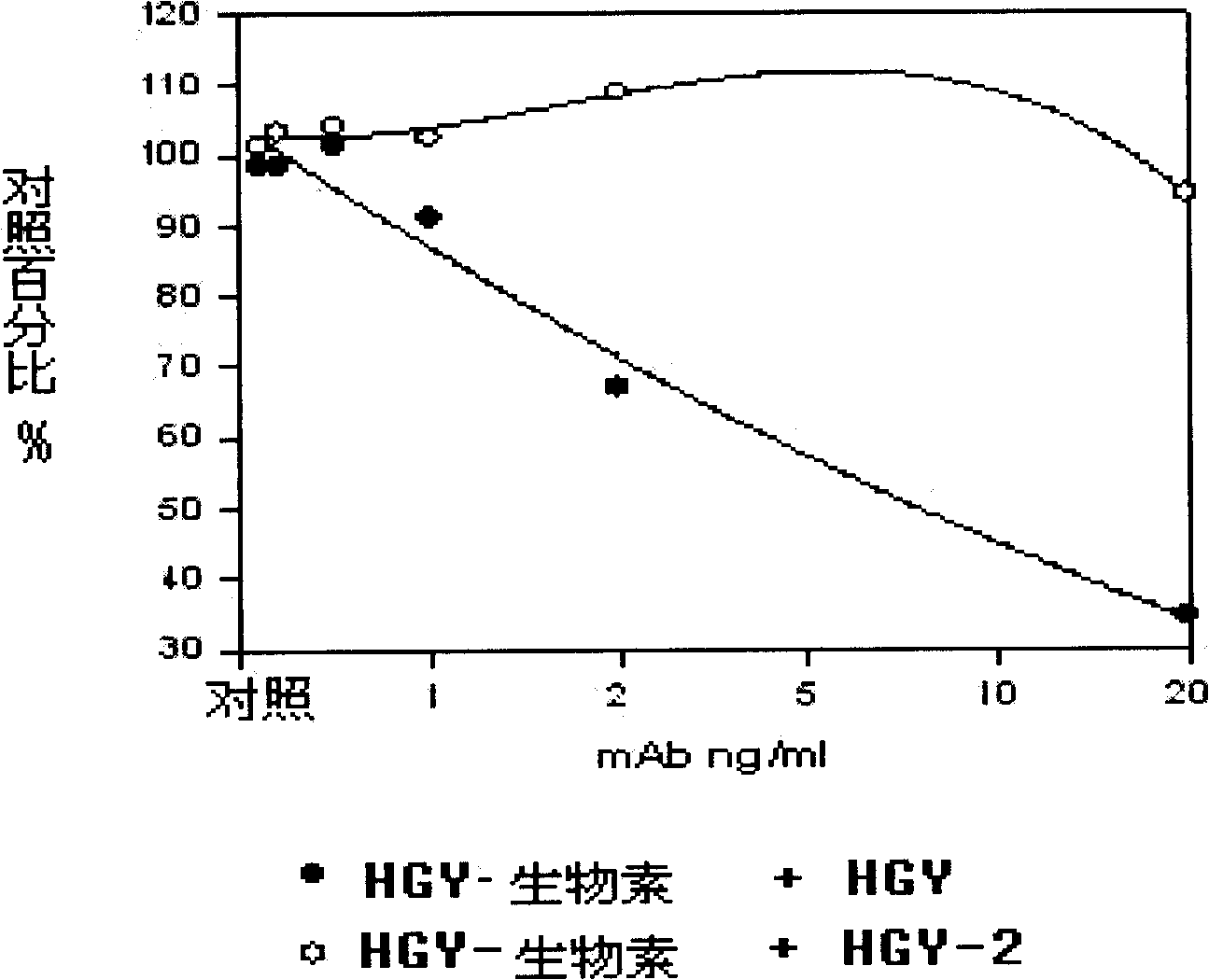
[0055] The kit uses two anti-HLA-G protein molecule-specific monoclonal antibodies (HGY and HGY-2). These two monoclonal antibodies can bind to different antigenic epitopes of HLA-G protein molecules. Antibodies were stored at -80°C.
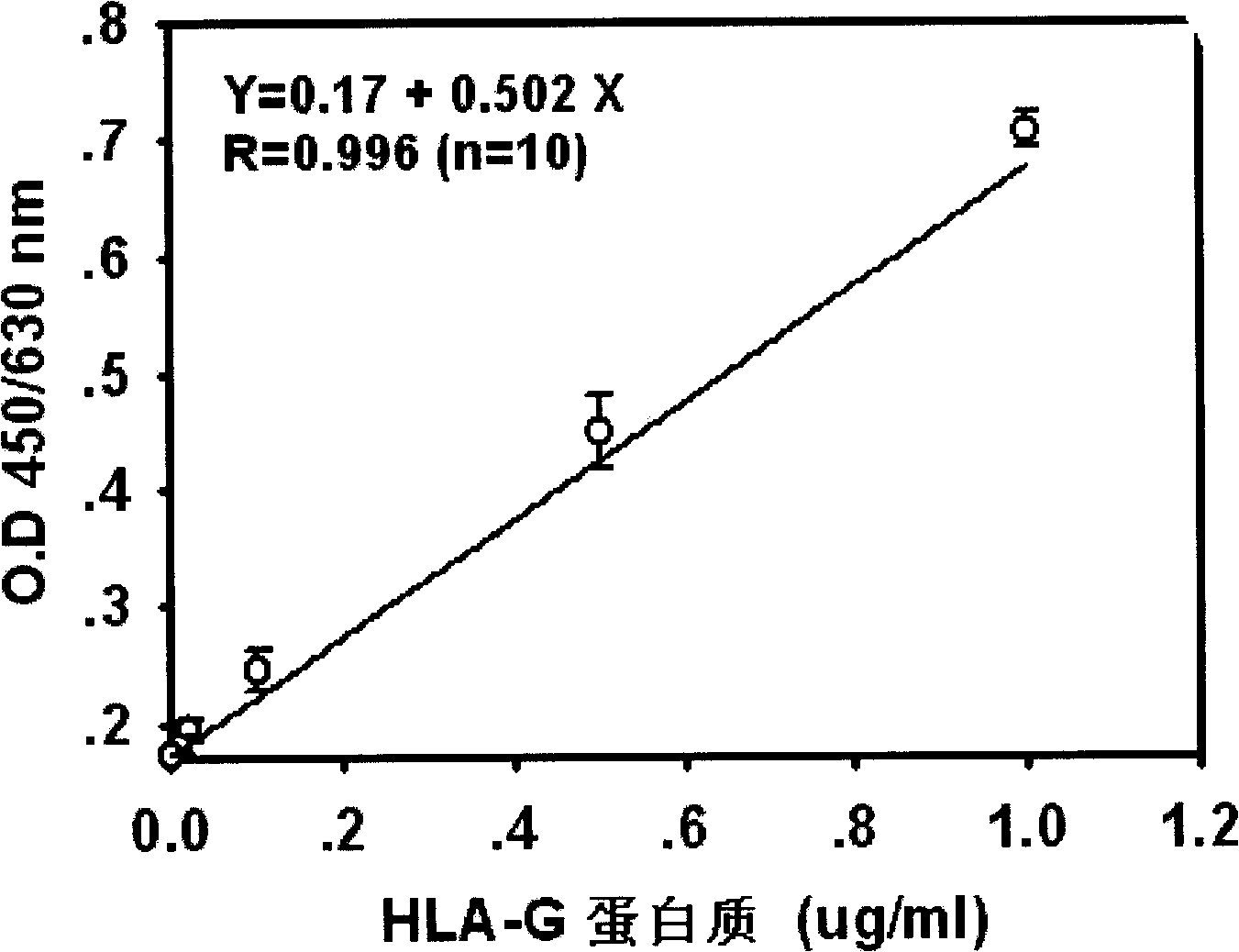
[0056] 2. Preparation of HLA-G protein standard:
[0057] The HLA-G protein standard used in the kit is obtained from the collected induced abortions by immunoaffinity chromatography using an anti-HLA-G monoclonal antibody (4H84 that has been commercialized or Ye Shangmian’s patent: HGY in ZL 2004 10040919). It is obtained by separating and purifying the extract of stage 1 placenta tissue, and its purity is greater than 95%. The method of gene recombination can also be used to clone and establish a cell line expressing HLA-G protein, and then use anti-HLA-G antibody immunoaffinity chromatography to separate and pu...
Embodiment 3
[0073] Example 3 Characteristics of the HLA-G enzyme-linked immunoassay kit of the present invention
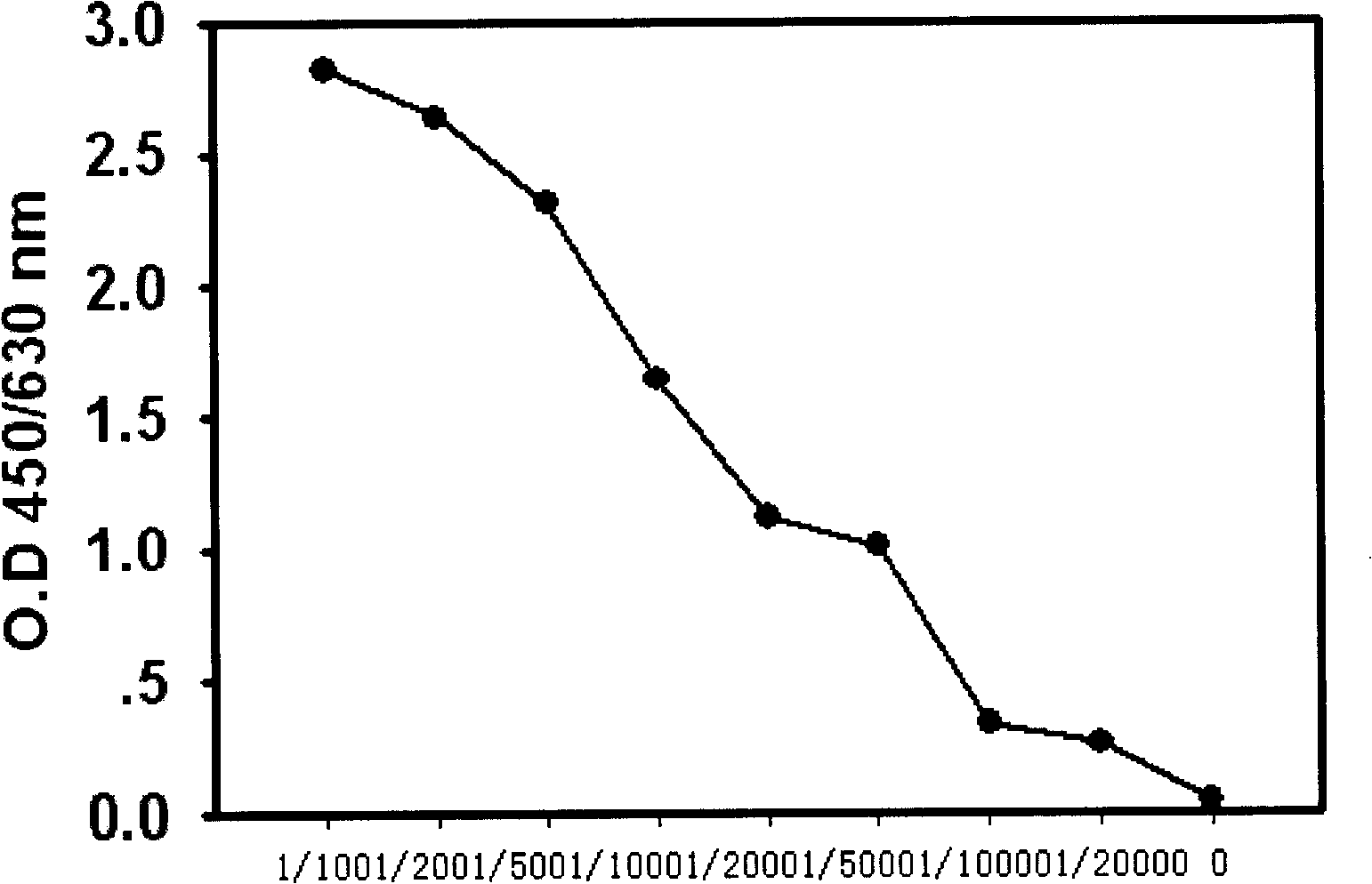
[0074] The present invention first establishes a sandwich type enzyme-linked immunoassay kit for detecting plasma / serum soluble HLA-G, and verifies the specificity, sensitivity, accuracy and repeatability of the kit.
[0075] ● image 3 A standard curve of 10 HLA-G ELISA assays is shown. The results show that the Sandwich ELISA kit established by the present invention has a sensitivity of 10 ng / ml and high repeatability (the average intra-assay and inter-assay errors of each standard point are less than 10%; Table 2). In each batch of testing, three doses of positive control samples were set up for the HLA-GELISA. It can also be seen from Table 2 that the intra-assay and inter-assay errors of the positive control samples are both less than 10%; further confirming the reproducibility of the kit.
[0076] Table 2. Reproducibility of HLA-G enzyme-linked immunohistochemical ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com