Catalyst for ethylene oxide production, method for producing the same, and method for producing ethylene oxide
A technology for the manufacture of ethylene oxide, applied in catalyst activation/preparation, organic chemical methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve problems such as unclear details, and improve catalyst life Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
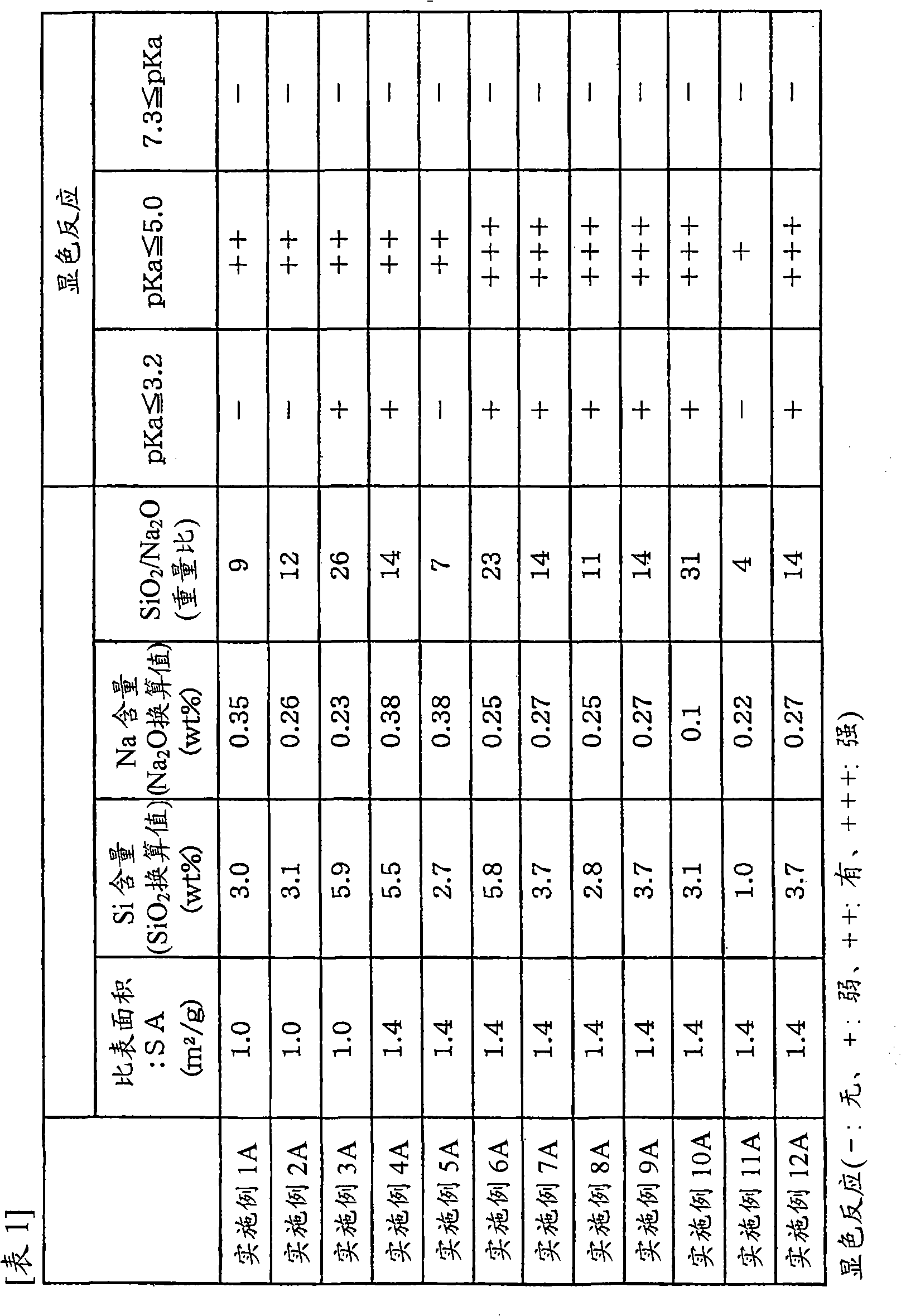
[0071] (1) Determination of the acidity and alkalinity of the carrier:
[0072] For α-alumina support (surface area 1.0m 2 / g, water absorption 35.7% by weight, SiO 2 3.0% by weight, Na 2 O 0.35 wt%, SiO 2 / Na 2 O weight ratio is 9, the shape is the ring of 8mmφ×8mm) carry out the measurement of acidity and alkalinity.
[0073] The use of methyl yellow with a pKa of 3.2 or less does not cause a red color reaction, while the use of methyl red with a pKa of 5.0 or less shows a red color reaction. In addition, the use of bromothymol blue with a pKa of 7.3 or more did not cause a blue color reaction. From the above, it can be seen that the above carrier has an acid ionization constant of 3.2<pKa≤5.0, and does not have an alkali ionization constant of pKa≥7.3.
[0074] (2) Pretreatment of the carrier:
[0075] 100g of the above-mentioned α-alumina carrier was impregnated with 0.156g cesium carbonate (Cs 2 CO 3 ) and 1.69g lithium carbonate (Li 2 CO 3 ) in a 200ml aqueou...
Embodiment 2A~12A
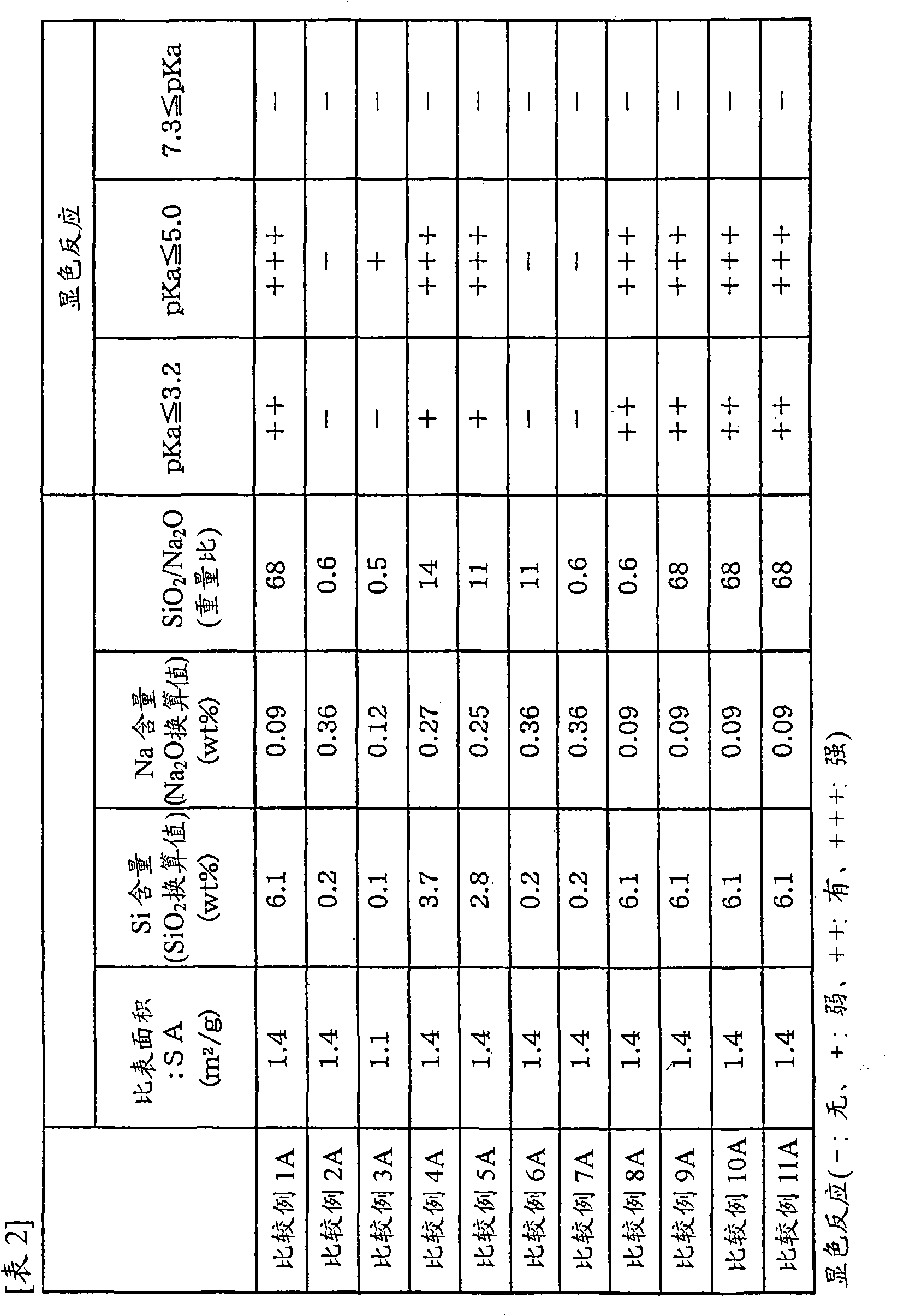
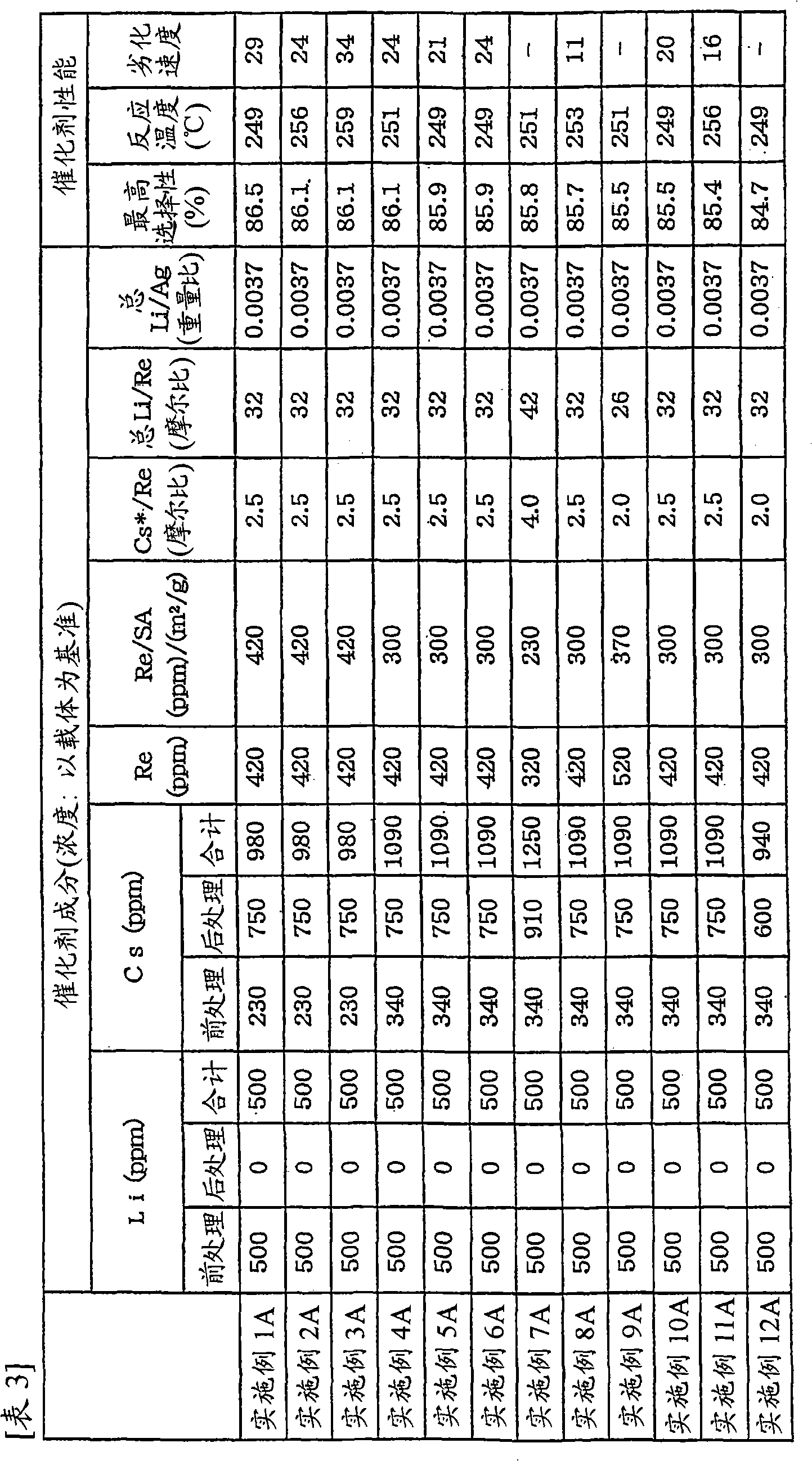
[0082] Examples 2A to 12A and Comparative Examples 1A to 11A
[0083] Carry out pretreatment in the mode of embodiment 1A, difference is: use the carrier with physical properties shown in table 1 and table 2, and change the amount of lithium carbonate and cesium carbonate, make the content of Li and Cs during pretreatment as table 3 and Table 4 shows. Then, prepare the "silver amine complex solution" identical to the composition in Example 1A, but in the "Preparation of Ag Catalyst" in Example 1A, change the concentration of cesium nitrate and ammonium perrhenate to obtain Cs and Catalysts with Re content as shown in Table 3 and Table 4. Also, the Ag content (based on the support) in any of the catalysts was 13.6% by weight. Then, ethylene oxidation reaction was carried out in the same manner as in Example 1A using various catalysts. Table 3 and Table 4 show the catalyst composition and catalyst performance.
[0084]
[0085]
[0086]
[0087]
[0088] It can b...
Embodiment 1B
[0095] (1) Pretreatment of the carrier
[0096] Carry out by the operation similar to embodiment 1A, difference is: use surface area 1.4m 2 / g, water absorption 41.6% by weight, SiO 2 2.8% by weight, Na 2 O 0.25 wt%, SiO 2 / Na 2 The ring-shaped α-alumina carrier with O weight ratio of 11 and shape of 8mmφ×8mm was changed, and the amount of lithium carbonate and cesium carbonate was changed so that the contents of Li and Cs during pretreatment were as shown in Table 6, thereby preparing the impregnated Carrier for Li and Cs components. The Li content in the support was 600 ppm, and the Cs content was 340 ppm. Table 5 shows the physical properties of the carriers used.
[0097] (2) Preparation of silver amine complex solution:
[0098] The same operation as in Example 1A was carried out to prepare a silver amine complex solution.
[0099] (3) Preparation of Ag catalyst:
[0100]The same operation as in Example 1A was carried out to obtain a catalyst. The contents of A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com