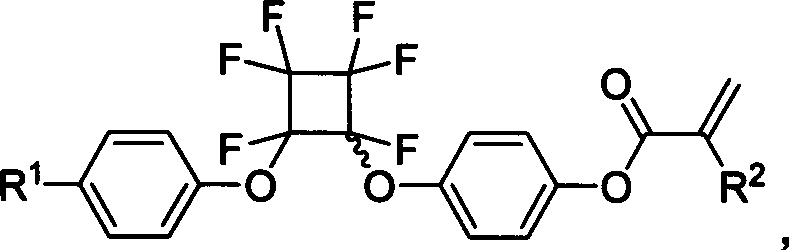
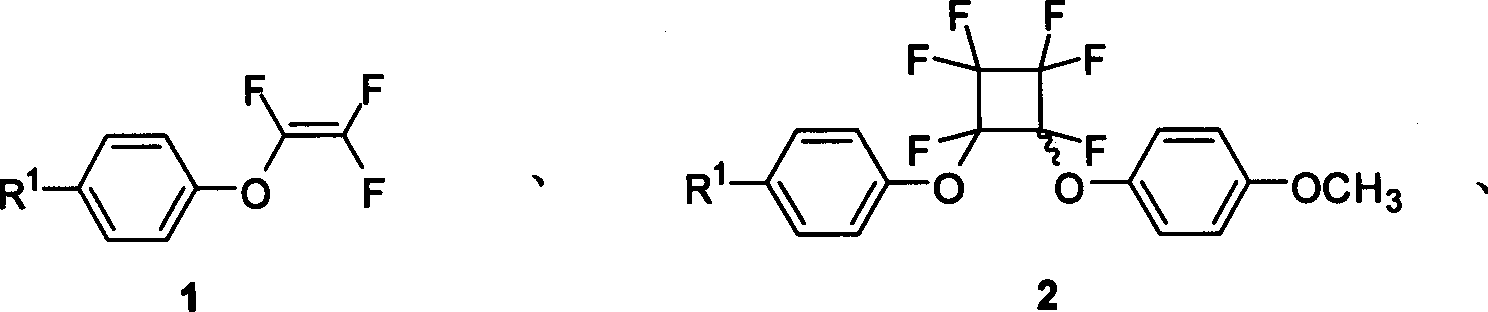
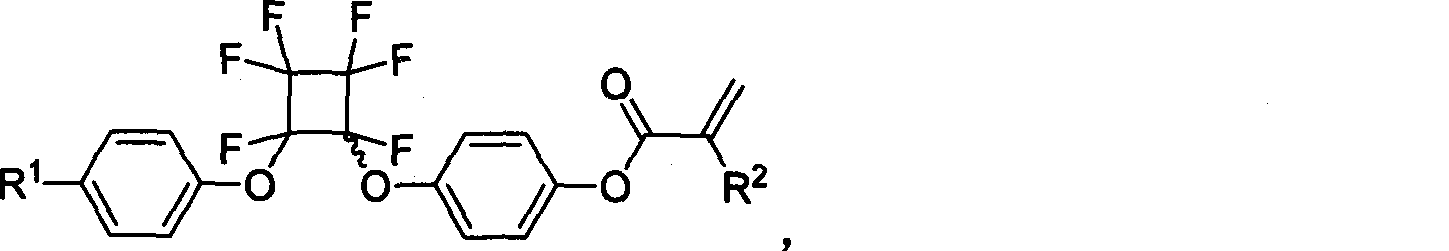
Functional acrylic esters monomers containing perfluorocyclobutane aryl-ether unit, preparation method and application
A technology of perfluorocyclobutyl aryl ether and acrylate, which is applied in the field of functional acrylate monomers and can solve the problems of limited application scope, limited design and synthesis, few types of polymers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Synthesis of 4-methyl-(2-bromotetrafluoroethoxy)benzene 7
[0026]
[0027] Add p-cresol (108 g, 1 mol) and dimethyl sulfoxide (DMSO) (700 mL) into a 1000 mL three-necked flask equipped with a water separator and a reflux condenser, and stir to dissolve the phenol. After decompressing with an oil pump for 30 minutes, nitrogen was bubbled into the solution for 30 minutes to remove oxygen in the solution. Under nitrogen protection, potassium hydroxide (68 g, 1 mol, 82%) was added. After bubbling nitrogen through the solution for 30 min, freshly distilled toluene (200 mL) was added. Under the protection of nitrogen, the temperature was raised to reflux the system, and the water layer in the water separator was continuously separated. After about 24 hours of reflux, when there is no more water layer in the water separator, stop heating and cool at room temperature. After the system is cooled to room temperature, quickly remove the water separator and replace...
Embodiment 2
[0029] Example 2 Synthesis of trifluorovinyl (4-methylphenyl) ether 8
[0030]
[0031]Install a reflux condenser on the 1000mL three-necked bottle, and add zinc powder (40g, 0.72mol). After replacing the nitrogen with an oil pump, it is baked with a gas fire, and then filled with nitrogen. repeat three times. After cooling to room temperature, 600 mL of freshly distilled acetonitrile was added. Compound 7 (156 g, 0.55 mol) was added in the dropping funnel. The temperature of the oil bath was raised to 110°C to reflux the acetonitrile. Then compound 7 was slowly added dropwise, and the dropwise addition was completed within 4 h. During the dropwise addition, acetonitrile was refluxed vigorously. After the dropwise addition, react at 110°C for 10h. Stop heating, and let it cool down at room temperature. The generated salt precipitated in the lower part of the three-necked flask, and the supernatant was poured out; the remaining reactants were washed with water, extrac...
Embodiment 3
[0033] Synthesis of Example 3 Compound 9
[0034]
[0035] Add compound 8 (18.8g, 100mmol) and trifluorovinyl (4-methoxyphenyl) ether (20.4g, 100mmol) into a 250mL dry three-necked flask, replace the nitrogen three times and then bubble with nitrogen for 20min , Put into 180 ℃ oil bath and stir for 24h. After cooling at room temperature, flash column chromatography gave 18.82 g of a colorless transparent liquid with a yield of 48%.
[0036] 1 H NMR (300MHz, CDCl 3 ): δ 2.31(s, 3H), 3.79(s, 3H), 6.83(d, J=7.2Hz, 2H), 7.03-7.13(m, 6H). 19 F NMR (282MHz, CDCl 3 ): δ-131.83, -131.63, -131.30, -131.03, -130.39, -130.25, -129.81, -129.59, -129.46, -129.02, -128.78, -128.67, -127.51. 13 C NMR (75MHz, CDCl 3 ): δ 20.6, 55.6, 114.6, 117.3, 118.2, 119.9, 123.0, 130.1, 134.8, 135.1, 146.1, 150.4, 157.0.IR(KBr): 3006, 2956, 2840, 1611, 1507, 1466, 1319, 1249 1193, 1118, 1036, 962, 829cm -1 .MS(EI) m / z(% relative intensity): 65(26), 77(45), 91(60), 107(26), 123(50), 157(22), 204...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com