T lymphocyte for screening and activating dormant infection HIV-1 compound and preparation thereof
A latent infection, lymphocyte technology, applied in the field of genetic engineering and cell engineering, can solve problems such as unsatisfactory results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation method of lentiviral vector
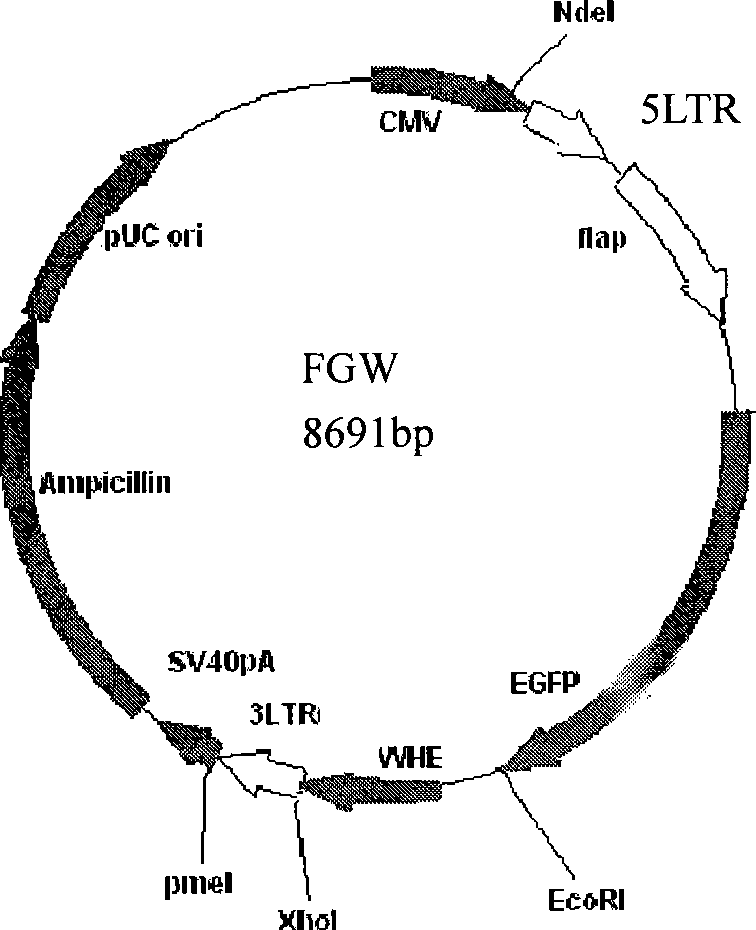
[0032] The lentiviral vector FUGW was digested with PacI+BamhI, the 8691BP fragment was recovered, ligated with T4 ligase, and the transformed bacterial clone was extracted for DNA sequence analysis, and finally the FGW plasmid with the deletion of the ubiquitin regulatory region was obtained.
Embodiment 2
[0033] Example 2 Preparation of lentiviral vector
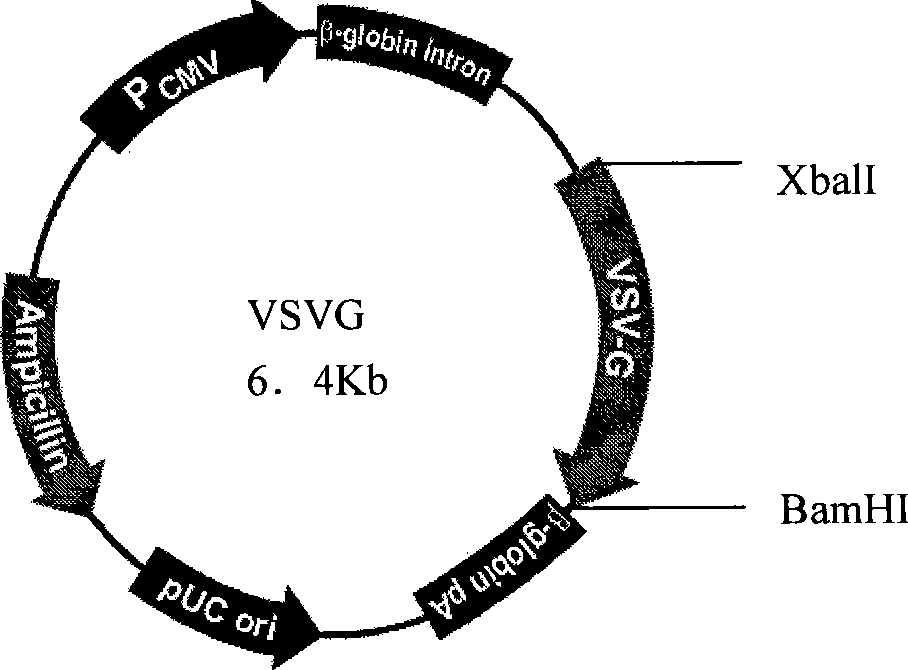
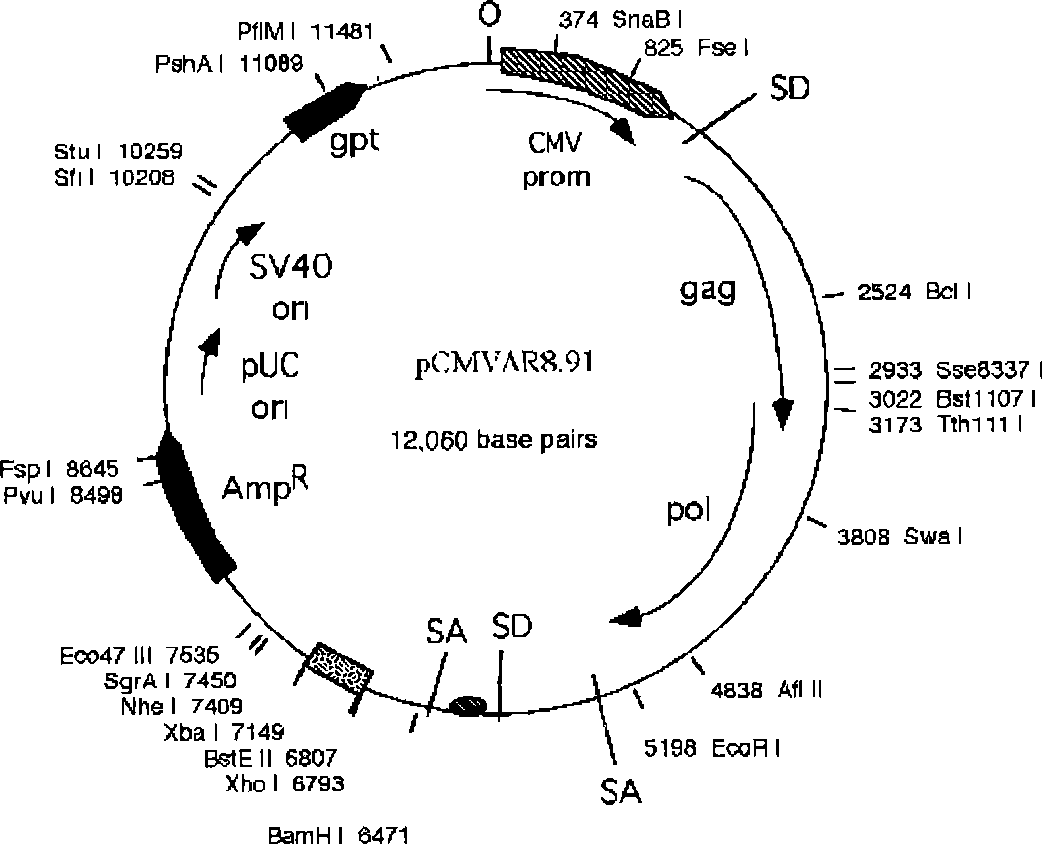
[0034]The FGW vector was mixed with the packaging construct plasmid CMVΔ8.9 and the envelope plasmid (VSVG) at a mass ratio of 2:1:1. Use liposome LipofectamineTM to transfect on 293T cells, observe under a fluorescent microscope after 24-48 hours, collect the virus supernatant after a large amount of fluorescence appears, and collect the collected virus supernatant after concentration for equipment or use immediately. Recombinant lentivirus activity During the measurement, the concentrated virus stock solution was diluted in different proportions, and the fluorescence count was performed under a fluorescence microscope after infecting the cells for 48 hours to determine the titer. FGW vector served as a control. The results showed that the site-specific integration lentivirus titer was 6.8X10 -7 TU / ML.
Embodiment 3
[0035] Example 3 Analysis of cells infected by lentivirus system
[0036] The above 1 ul lentiviral system FGW- (packaged by plasmid CMVΔ8.9) was infected with the Jurkat cell line on a 6-well plate. After 72 hours, observation under a fluorescent microscope showed that Jurkat cells showed green fluorescence; the infection efficiency was 82.1%. Non-fluorescing cells were harvested and subjected to single cell clonal expansion in 96-well plates.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com