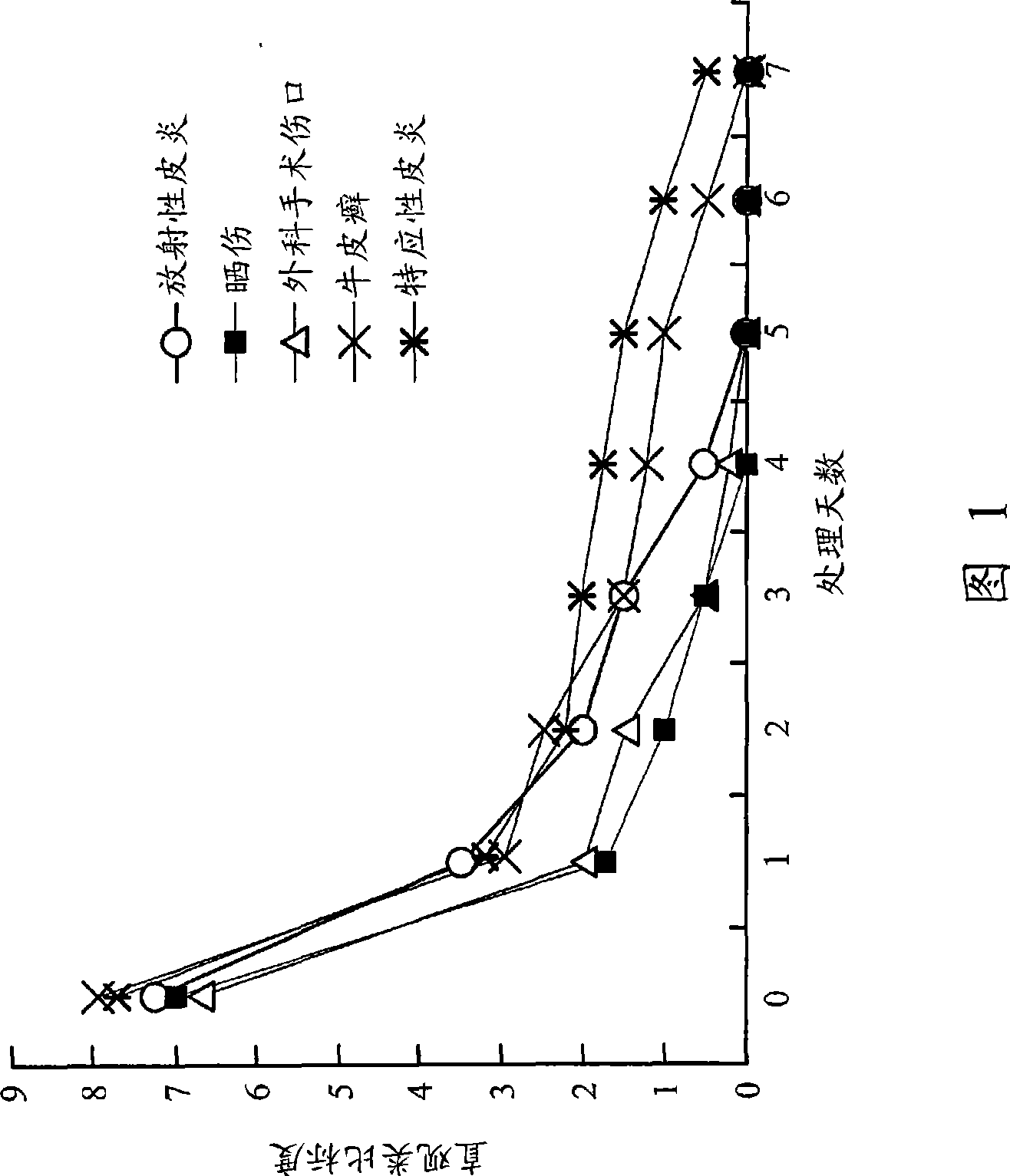
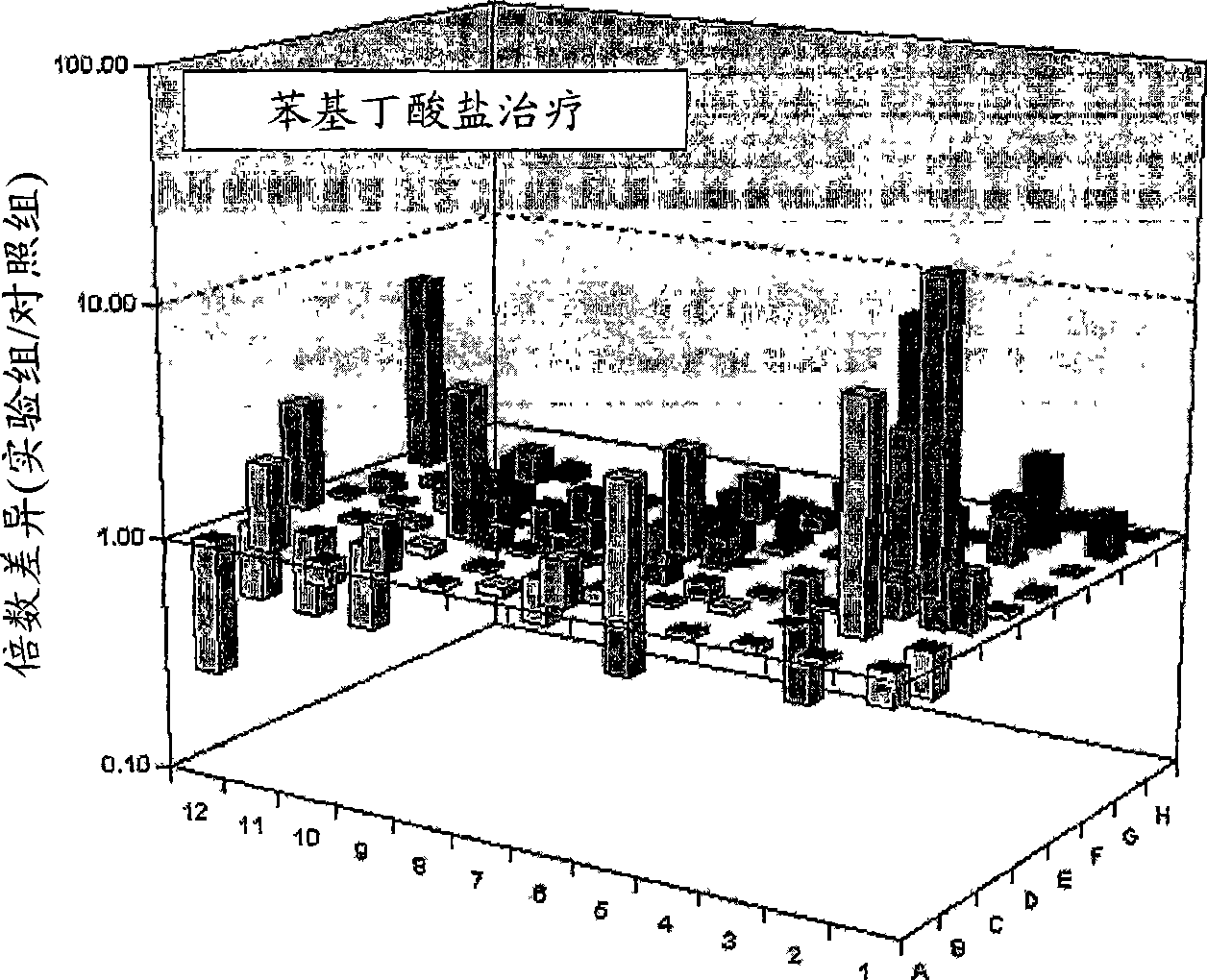
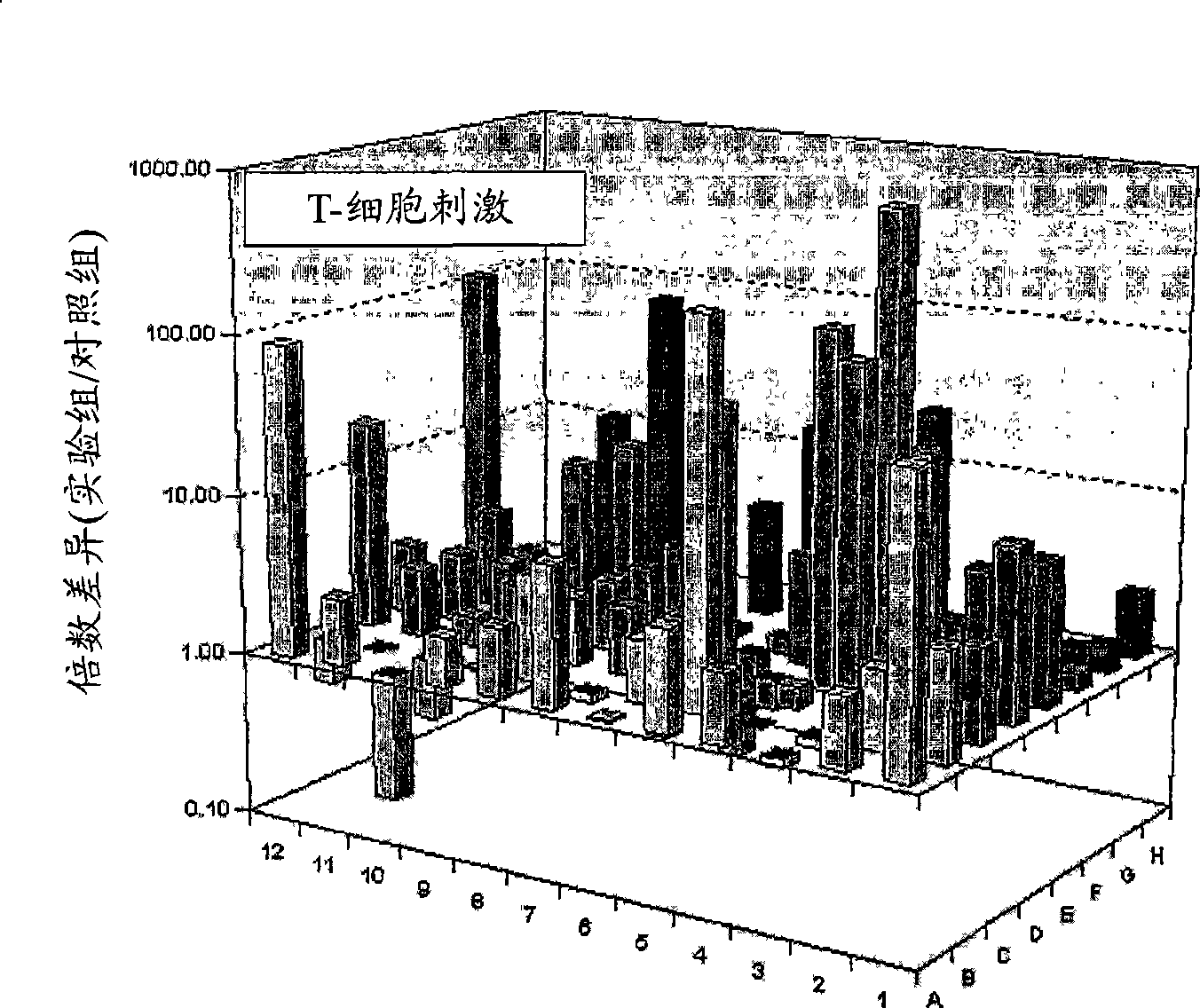
Pharmaceutical composition for easing pruritus
A composition and technology for pruritus, applied in the directions of drug combination, drug delivery, active ingredients of heterocyclic compounds, etc., can solve problems such as patients who are not suitable for chronic pruritus and have side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0024] 1. Preparation of various types of oily ointments, creams and gels
[0025] A. Preparation of phenylbutyrate oily ointment
[0026] Mix 65 grams of white petrolatum (Riedel-de Haen), 15 grams of cetyl alcohol (Riedel-de Haen), 260 grams of soft paraffin (Merck), 155 grams of liquid paraffin (Merck), and 5 grams of 4-phenylbutyrate (Merck) were mixed in a beaker and heated to 70°C to form a paste. The paste was stirred at 400 rpm for 1 hour and cooled to room temperature.
[0027] B. Preparation of Phenylbutyrate Emulsion
[0028] First ingredient: 70 grams 20 grams 10 grams Coster 15 grams Myriyol 15g Coster and 15 grams of GMS (both available from local suppliers) were mixed in a beaker and heated to 70°C.
[0029] Second component: 5.739 grams sodium 4-phenylbutyrate (Triple Crown America, Inc.), 0.125 grams methylparaben (Merck), 0.075 grams propylparaben (Merck ), and 149.061 grams of deionized water were mixed in a beaker and heated to 70°C.
[003...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com