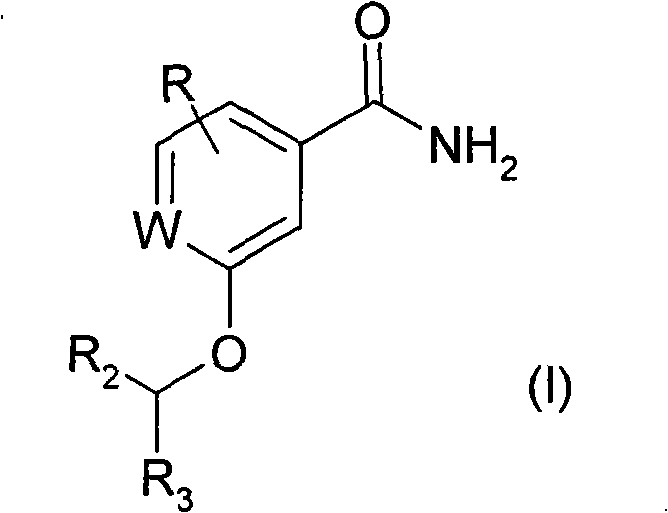
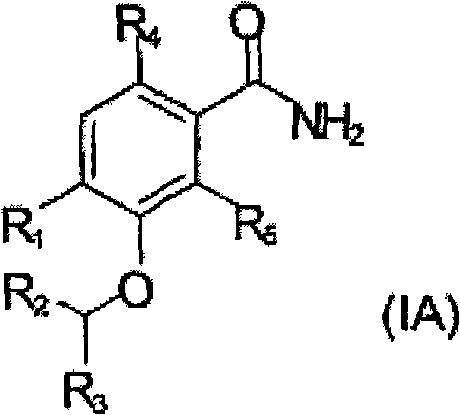
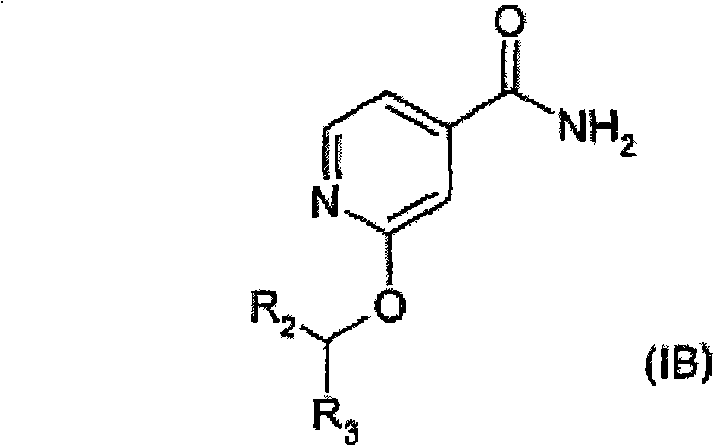
Antibacterial agents
A solvate, bacterial infection technology, applied in the field of antimicrobial agents, which can solve the problems of undesired pharmacological properties, low potency, unknown specificity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] Example 1: 3-Nonyloxy-benzenecarboxamide.
[0180]
[0181] Add K 2 CO 3 (302mg, 2.19mmol, 1.5eq) and NaI (43.5mg, 0.29mmol, 0.2eq). The suspension was stirred for 5 minutes before n-nonyl chloride (0.32ml, 1.61mmol, 1.1eq) was introduced. The resulting mixture was heated to 60°C for 16 hours. Afterwards, the reaction was cooled to room temperature and partitioned between EtOAc and water. The organic phase was separated, washed with additional water (x2), dried (MgSO 4 ), filtered and concentrated in vacuo to give a colorless solid. For 3-n-nonyloxybenzamide, the colorless solid was stirred with MeOH (~0.5 ml) for 5 minutes [NB: 3-n-nonyloxybenzamide was partially dissolved in MeOH], then filtered to give The desired compound was obtained as a colored solid (116 mg, 30%). HPLC-MS (Method 3): m / z 264 [M+H] + , Rt = 1.80 min. 1 HNMR(d 6 -DMSO) δ=7.95(s, 1H), 7.44-7.31(m, 4H), 7.06(ddd, J=8Hz, J=2Hz, J=1Hz, 1H), 3.99(t, J=6.5Hz, 2H ), 1.72 (quintet, J=6.5Hz...
Embodiment 2-44
[0187] Examples 2-44 (Table A)
[0188] Examples 2-44 were prepared according to Method B, Scheme 2
[0189]
[0190]
[0191]
[0192]
[0193]
[0194]
[0195]
[0196]
[0197]
[0198]
[0199]
[0200]
[0201]
[0202] List of product compound names; Examples 2-44:
[0203] Example
2
3-Propoxybenzenecarboxamide
3
3-Isopropoxybenzenecarboxamide
4
3-(cyclopropylmethoxy)benzenecarboxamide
5
3-(pentyloxy)benzenecarboxamide
6
3-(allyloxy)benzenecarboxamide
7
3-butoxybenzenecarboxamide
8
3-(Hexyloxy)benzenecarboxamide
9
3-(2-Methoxyethoxy)benzenecarboxamide
10
3-(4-phenoxybutoxy)benzenecarboxamide
11
3-[(2-Methyl-2-propenyl)oxy(oxy)]benzenecarboxamide
12
3-(7-octenyloxy)benzenecarboxamide
13
3-(Isoamyloxy)benzenecarboxamide
14
3-[(4-Methylpenty...
Embodiment 45
[0208] Example 45 3-[(Z)-5-Decenyloxy]benzenecarboxamide
[0209]
[0210] In a polymer-supported suspension of triphenylphosphine (1.4 g, 3 mmol, 2.15 mmol / g based on loading [purchased from Argonaut], 1.5 equivalents) swollen in THF (20 ml) at room temperature, Diisopropylazodicarboxylate (0.47ml, 2.4mmol, 1.2eq) was added. The mixture was shaken for 5 minutes, then 3-hydroxybenzamide (274mg, 2mmol, 1eq), triethylamine (0.28ml, 2mmol, 1eq) and cis-5-decenol (313mg, 2mmol, 1eq) were added. equivalent). The resulting suspension was shaken at room temperature for 16 hours, then filtered. The resin was washed with additional THF (x 3), then the combined filtrate and washings were concentrated under reduced pressure to give the crude product as a colorless semi-solid. Column chromatography on a silica gel column eluting with EtOAc / hexanes (20%-40% gradient) afforded the desired compound (390 mg, 71%) as a white solid, mp 98-100 °C. HPLC-MS (Method 1): m / z 276 [M+H] + ,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com