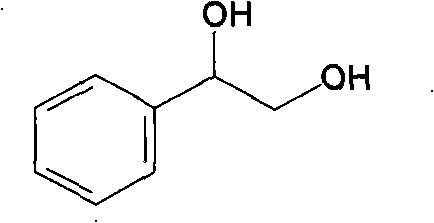
Method for preparing (R)-styrene glycol by changing coenzyme specificity and stereoselectivity via site-directed mutagenesis
A technology of phenylethylene glycol and its construction method, which is applied in the field of biocatalytic asymmetric transformation, and can solve the problems of consumption, insufficient substrate concentration, and low optical purity of products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Cultivation of the recombinant strain E.coli / pETCPADH: The recombinant strain E.coli / pETCPADH has been published in [Applied and Environmental Microbiology 200773(11):3759-3764]. LB medium was used, and its composition was: tryptone 1%, yeast extract 0.5%, NaCl 1%, pH 7.0. Ampicillin (100 μg / mL) was added during cultivation. Add 1.5% agar powder to the solid medium. Pick a single colony of the positive clone and inoculate it in 3 mL of LB liquid medium containing 100 μg / mL ampicillin, and cultivate overnight at 37°C with shaking at 200 rpm. On the next day, take 1 mL of the culture solution and transfer it to 50 mL of LB liquid medium containing 100 μg / mL ampicillin, culture at 37°C, 200 rpm for about 12 hours, until the OD 600 is 0.6.
Embodiment 2
[0093] Obtaining the pETCPADH plasmid from the recombinant strain E.coli / pETCPADH: centrifuge the cell culture solution at 5,000rpm for 10min, collect the cell, and extract the plasmid using the plasmid extraction kit Mini-Plasmid Rapid Isolation Kit (Beijing Broadtech Biogene Technology Co., Ltd.) pETCPADH.
Embodiment 3
[0095] Use the recombinant plasmid pETCPADH containing (S)-specific carbonyl reductase as the PCR amplification reaction template, use primer 1 containing the BamHI restriction enzyme site, primer 2 containing the XhoI restriction enzyme site, and the middle two Segment Primer 3 and Primer 4,
[0096] Primer 1: 5'-ATC GGATCC GATGGGCGAAATCGAATCTTATTG-3',
[0097] Primer 2: 5'-TGACTCTCGAGTGGACACGTGTATCCACCGTC-3',
[0098] Primer 3: 5'-CCATTTGGTACAACGATGATCCAGC-3',
[0099] Primer 4: 5'-CTCATCAGCTGGATCATCGTTGTAC-3',
[0100] The scr6768 gene was amplified by SOE-PCR reaction, and its full length was 837bp;
[0101] Acquisition of upstream cDNA fragments and downstream cDNA fragments: use the recombinant plasmid pETCPADH as a template, and perform PCR reactions with primers 1 and 4, and primers 2 and 3, respectively. PCR reaction system: ddH 2 O 37μL, 10×Reaction Buffer 5μL, 25mmol / L dNTP 0.5μL, 50pmol / μL primers 1 and 4, or primers 2 and 3 each 1μL, recombinant plasmid pETCPA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com