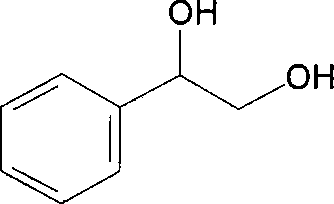
Method for preparing (R)-styrene glycol by employing asymmetric conversion of recombinant strain
A technology of phenylethylene glycol and recombinant strains, applied in microorganism-based methods, biochemical equipment and methods, bacteria, etc., can solve the problems of not using auxiliary substrates and the amount of recombinant cells being too much, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Induced expression culture: LB medium was composed of tryptone 1%, yeast extract 0.5%, NaCl 1%, pH 7.0. If necessary, ampicillin (50 μg / mL) was added before use, and 1.5% agar powder was added to the solid medium. A single colony of positive clones was picked and inoculated into 3 mL of LB liquid medium containing 50 μg / mL ampicillin, and cultured overnight at 37° C. with shaking at 200 rpm. Transfer 1 mL of culture medium to 50 mL of LB liquid medium containing 50 μg / mL ampicillin, and culture at 37°C with shaking at 200 rpm to OD. 600 About 0.6 mmol / L of inducer IPTG was added to the culture, and induction culture was performed at a culture temperature of 30°C.
Embodiment 2
[0059] Induced expression culture: the composition of LB medium is the same as that of Example 1. A single colony of positive clones was picked and inoculated into 3 mL of LB liquid medium containing 50 μg / mL ampicillin, and cultured overnight at 37° C. with shaking at 200 rpm. Transfer 1 mL of culture medium to 50 mL of LB liquid medium containing 50 μg / mL ampicillin, and culture at 37°C with shaking at 200 rpm to OD. 600 about 0.6. 1 mmol / L of inducer IPTG was added to the culture, and induction culture was performed at a culture temperature of 37°C.
Embodiment 3
[0061] In 1mL 0.1mol / L Tris-HCl buffer (pH8.0), add 1g / L substrate 2-hydroxyacetophenone, 0.1g / mL recombinant Escherichia coli wet cells, and auxiliary substrate isopropanol respectively 2.5%, after mixing, the reaction was shaken on a constant temperature shaker at 30°C for 48 hours. After the reaction, the mixture was centrifuged, and the supernatant was extracted. The optical purity of the product (R)-phenylethylene glycol was 86.2%, e.e., the yield was 81.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com