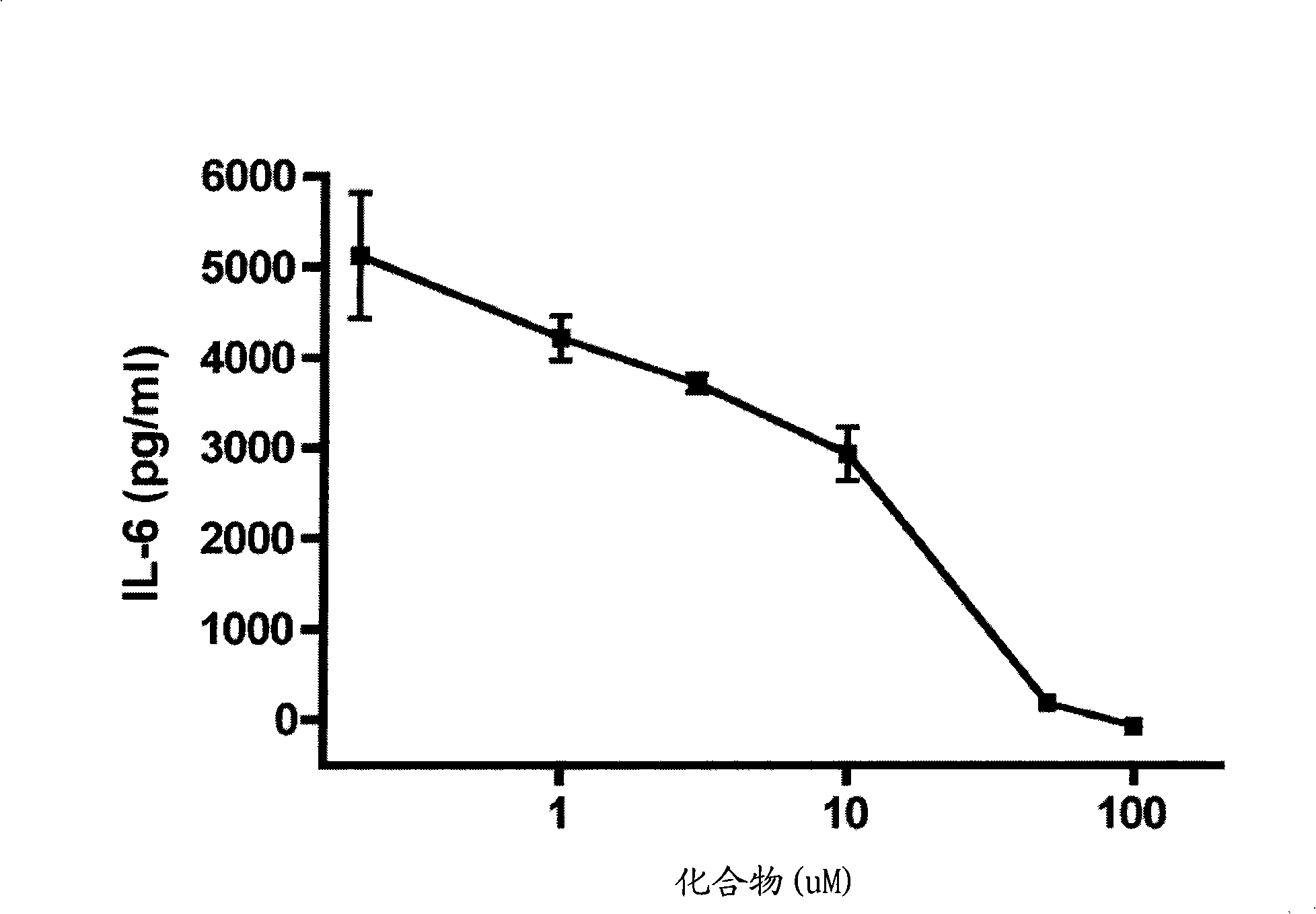
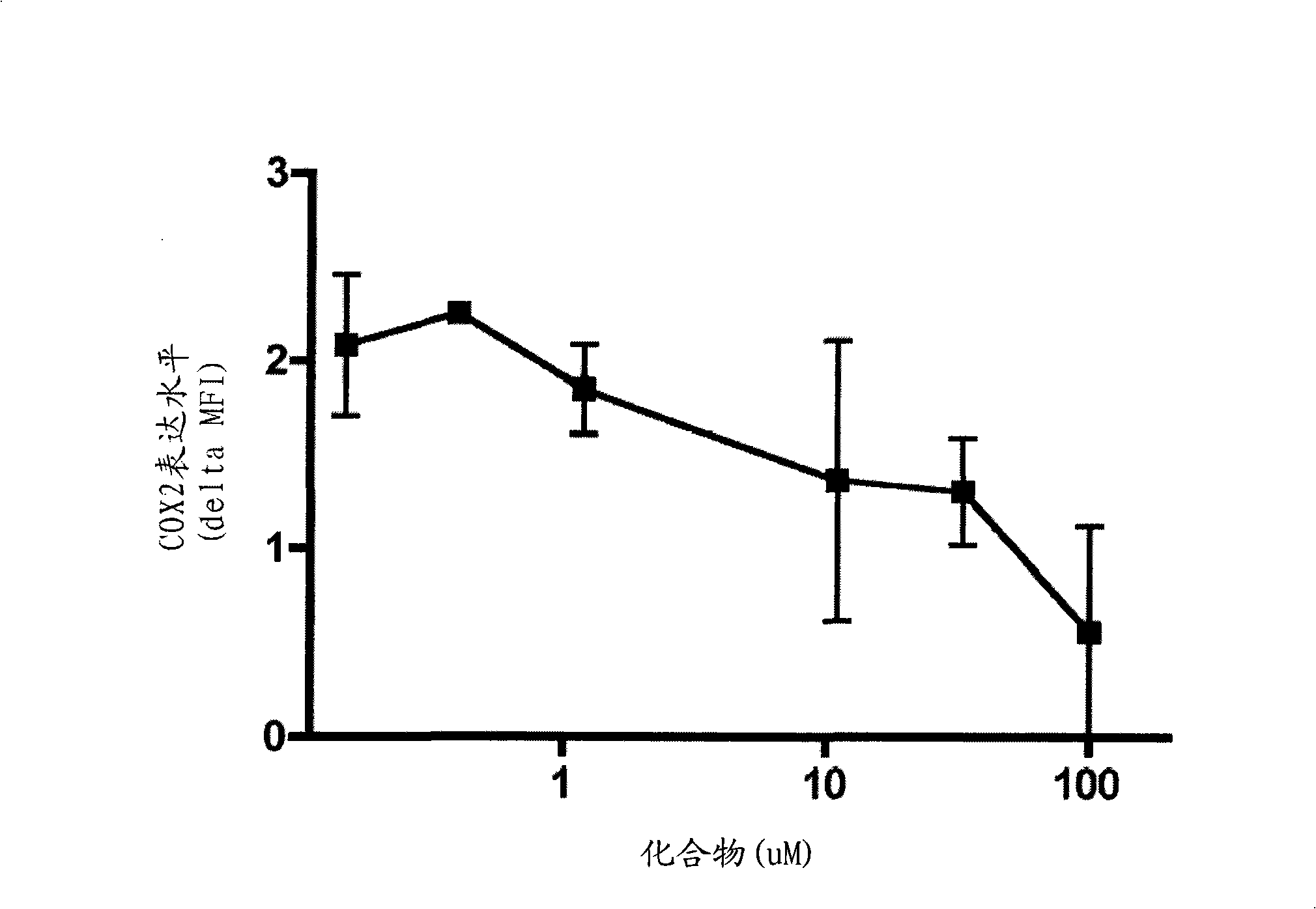
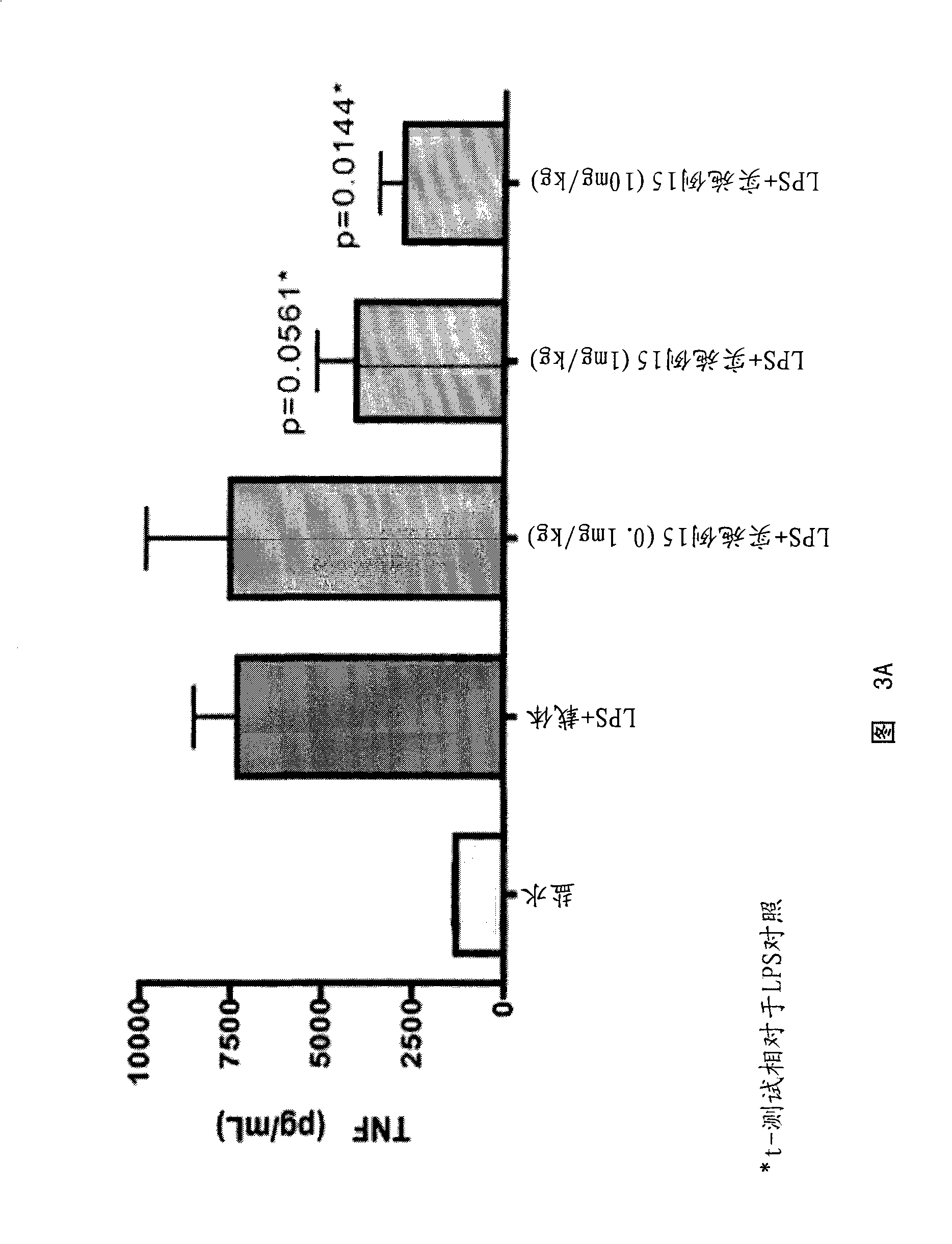
MIF inhibitors
A technology selected from, alkyl, applied in the field of treatment of MIF cytokines or biologically active diseases or diseases, inflammatory or cancerous diseases or diseases, and can solve the problem of incomplete effectiveness of glucocorticoids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0480]
[0481] Preparation of methyl 3-((2-oxo-2-(2-oxo-2,3-dihydro-1H-indol-5-yl)ethyl)thio)propionate (1)
[0482] (i) 5-Bromoacetyloxindole (US 5849710)
[0483] To a stirred suspension of anhydrous aluminum chloride (11.4 g, 85 mmol) in 1,2-dichloroethane (25 ml) at 0°C was added bromoacetyl bromide (5.9 ml, 68 mmol) dropwise. After 1 h, a suspension of oxindole (4.52 g, 34 mmol) in 1,2-dichloroethane (25 ml) was added and stirring was continued at 0 °C for 2 h, then at 50 °C for 3 h. The reaction mixture was cooled, poured onto ice / water (500ml) and filtered to give 5-bromoacetyloxindole (7.1g, 82%) as a beige solid which was used without further purification .
[0484] (ii) Methyl 3-((2-oxo-2-(2-oxo-2,3-dihydro-1H-indol-5-yl)ethyl)thio)propanoate (1)
[0485] To a suspension of 5-bromoacetyloxindole (4.15g, 16.3mmol) in N,N-dimethylformamide (20ml) was added methyl 3-mercaptopropionate (2.14ml, 19.6mmol) and diiso Propylethylamine (6.1 mL, 35 mmol), resulting in ...
Embodiment 2
[0489]
[0490] Preparation of 3-((2-oxo-2-(2-oxo-2,3-dihydro-1H-indol-5-yl)ethyl)thio)propionic acid (2)
[0491] A solution of the methyl ester (1) (3.0 g, 10.2 mmol) in concentrated hydrochloric acid (30 ml) was heated to reflux for 5 minutes and then cooled to room temperature to give a yellow precipitate. The solid was filtered, washed with water and recrystallized in methanol to give acid (2) (1.50 g, 52%) as a pale yellow solid, mp. 182-184°C (TLC: R F = 0.31 in SiO 2 above, 9:1 chloroform / methanol).
[0492] 1 H nmr (300MHz, d 6 -dmso) δ2.5, shielded, CH 2 ;2.65, t(7.1Hz), CH 2 ;3.54,s,H3;3.94,s,SCH 2 CO; 6.89, d(8.1Hz), H7; 7.82, s, H4; 7.87, d(8.4Hz), H6; 10.74, br s, NH; 12.21, br s, COOH.
[0493] ESI(+ve) m / z 280(M+H, 100%).ESI(-ve)m / z 278(M-H, 100%).
Embodiment 3
[0495]
[0496] Preparation of methyl 3-((2-oxo-2-(2-oxo-2,3-dihydro-1H-benzimidazol-5-yl)ethyl)thio)propionate (3)
[0497] (i) 5-(Chloroacetyl)-1,3-dihydro-2H-benzimidazol-2-one (WO 92 / 50070)
[0498] Under nitrogen, anhydrous aluminum chloride (7.5 g, 60 mmol) was powdered and then suspended in 1,2-dichloroethane (10 ml). The suspension was cooled to 0°C, and chloroacetyl chloride (3.6ml, 45mmol) was added dropwise. After stirring at 0°C for 30 minutes, 2-hydroxybenzimidazole (3.0 g, 22.4 mmol) and further 1,2-dichloroethane (5 ml) were added in portions. The reaction mixture was heated at 50-55 °C under nitrogen with vigorous stirring for 2 hours, during which time the blue-green suspension turned into a dark solution. After stirring at room temperature for 16 hours, the mixture was poured on ice (100 g) and the resulting gray precipitate was filtered. The resulting solid was washed with water, then ethyl acetate and dried under vacuum to give 5-(chloroacetyl)-1,3-di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com