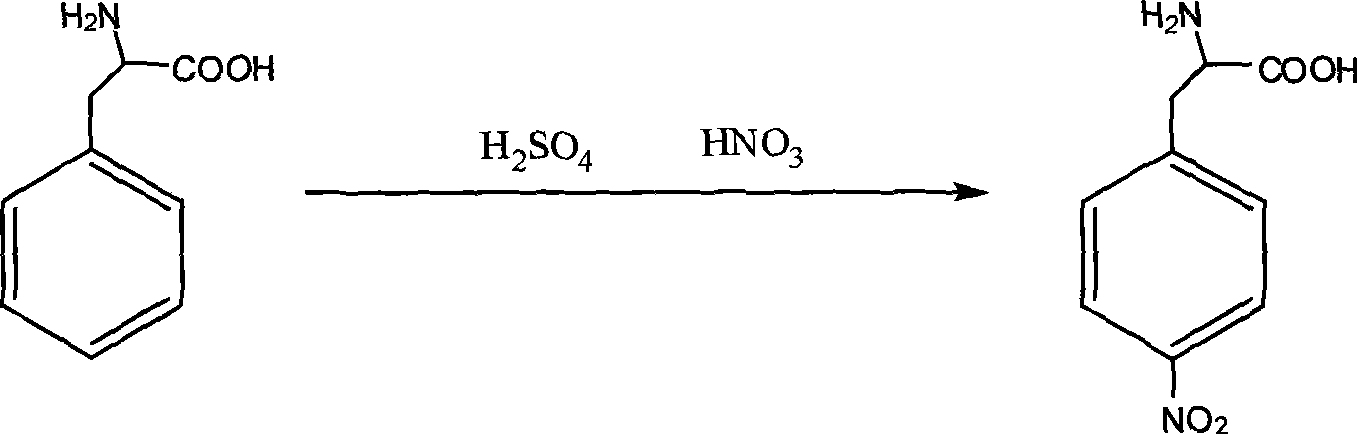
Synthesis method of L-p-nitrophenylalanine
A technology of nitrophenylalanine and a synthetic method, applied in the field of organic compound synthesis, can solve the problems of long reaction time, increased production cycle, slow reaction rate, etc., achieves short production time, high yield, overcomes production time long effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
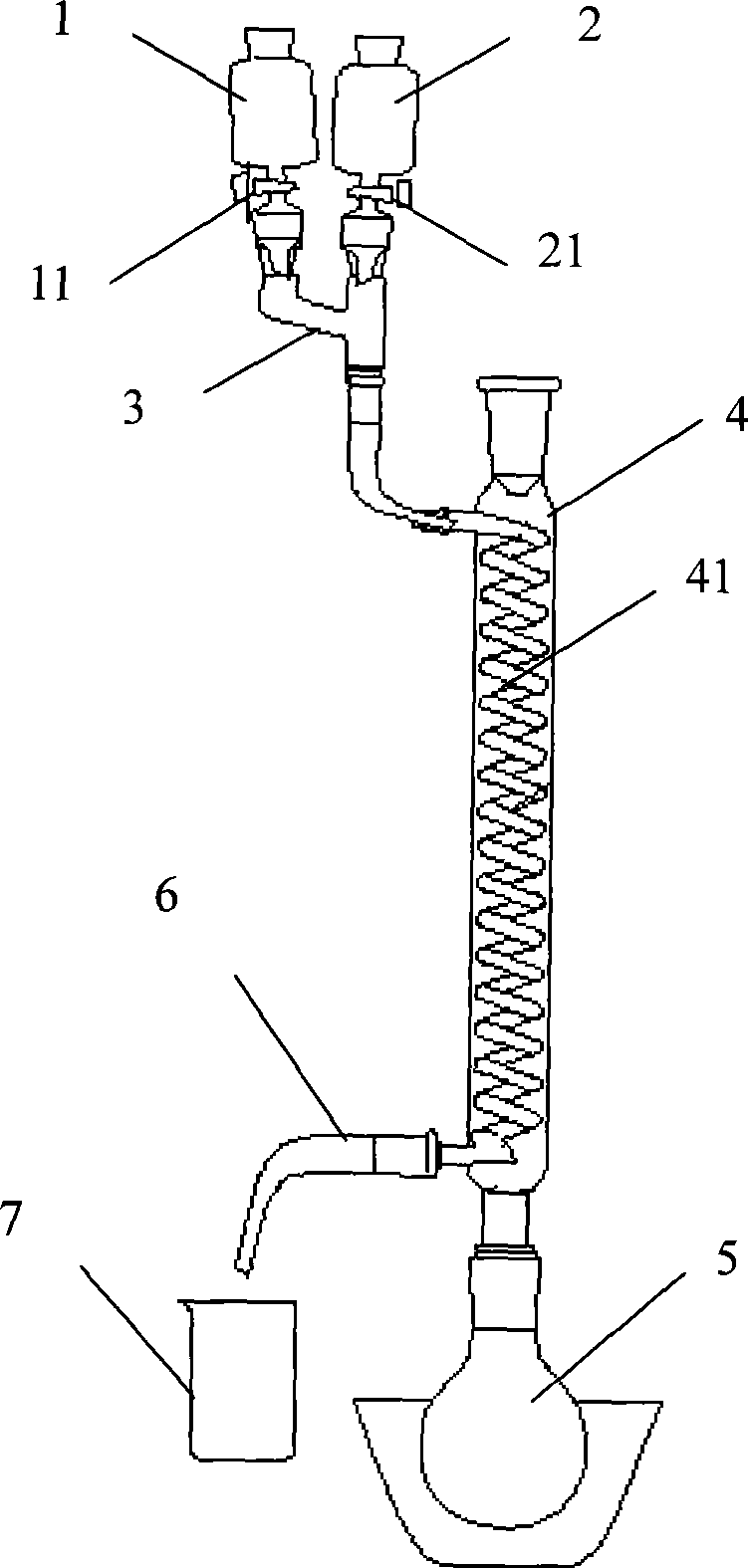
[0022] Embodiment 1, figure 1 A pipelined reaction device is provided, including liquid storage tank I1, liquid storage tank II2, premixing pipeline 3, coil reactor 4 containing coil 41, water bath heating device 5, discharge pipe 6 and product storage tank7. The liquid storage tank I1 is provided with a feed valve I11, and the feed valve I11 is used to control the flow rate of the material in the liquid storage tank I1; the liquid storage tank II2 is provided with a feed valve II21, and the feed valve II21 is used to control the liquid storage tank The flow rate of the material in II2.
[0023] The upper end of the premixing pipeline 3 is connected with the liquid storage tank I1 and the liquid storage tank II2 respectively, and the lower end of the premixing pipeline 3 is connected with the upper end of the coil 41 . One end of the discharge pipe 6 is connected to the lower end of the coil pipe 41 , and the other end of the discharge pipe 6 is aligned with the product stor...
Embodiment 2
[0025] Embodiment 2, a kind of synthetic method of L-p-nitrophenylalanine, carry out following steps successively:
[0026] 1) First, dissolve 8.3g (0.05mol) of L-phenylalanine in 20mL of concentrated sulfuric acid (98%w). After the L-phenylalanine is completely dissolved, put the solution in the storage tank I1 Inside. Put 20mL of concentrated nitric acid (64%w) in the storage tank II2. Turn on the water bath heating device 5, and control the temperature in the coil 41 to 50°C.
[0027] When the temperature in the coil pipe 41 reaches the above-mentioned set temperature (i.e. 50° C.), the feed valve I11 and the feed valve II21 are opened, and the solution in the liquid storage tank I1 and the concentrated nitric acid in the liquid storage tank II2 are fed at the same rate. The speed flows into the premixing pipeline 3, so that the two are pre-mixed to form a mixed liquid.
[0028] The mixed solution in the premixing pipeline 3 flows into the coiled tube 41 in the coiled tu...
Embodiment 3
[0030] Embodiment 3, a kind of synthetic method of L-p-nitrophenylalanine:
[0031] Change the reaction temperature in step 1) of embodiment 2 into room temperature of 25° C., and all the other contents are the same as in embodiment 2. The product yield finally obtained is 55%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com