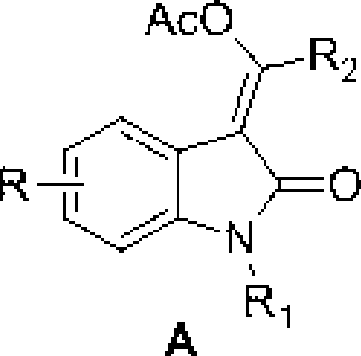
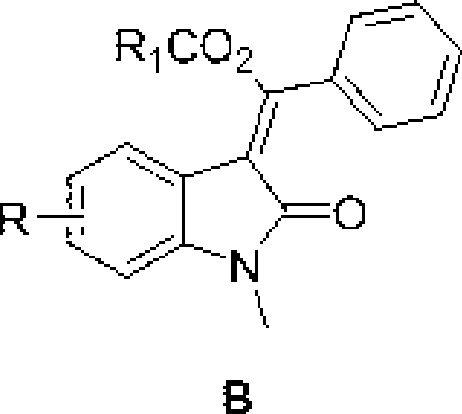
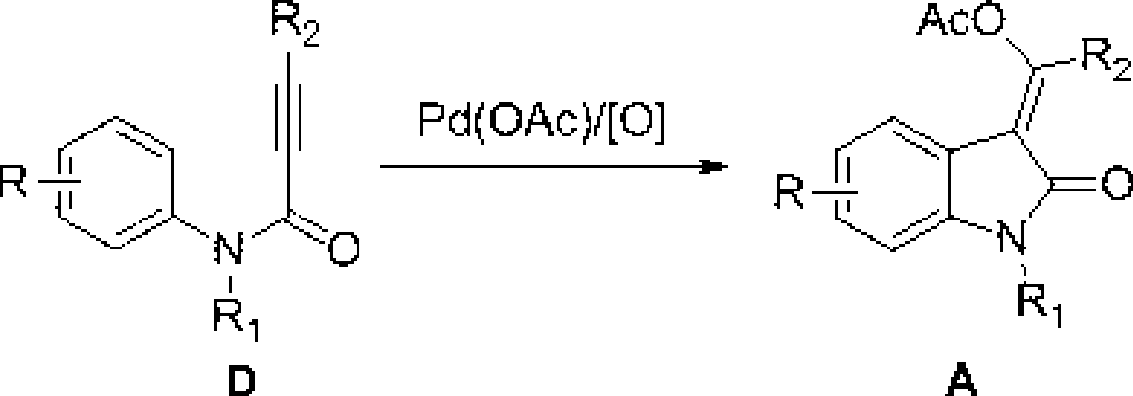3-methylene-indol-2-one derivates and preparation method thereof
A technology of methylene indole and its derivatives, which is applied in the field of 3 methylene-indol-2-one derivatives and its preparation, and can solve problems such as limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Take the preparation of (E)-1-methyl-3-(1`-phenyl-1`-acetoxy)methylene-indol-2-one as an example:
[0033] The compound N-methyl-N-phenyl-phenylpropanamide (0.047g), Pd(OAc) 2 (0.0045g), PhI(OAc) 2 (0.0773 g) was placed in a 25 mL round bottom flask, then HOAc (3 mL) was injected and the flask was sealed. React at a temperature of 60°C-100°C, but preferably at a temperature of 80°C. After the reaction (usually about 2 hours), add ethyl acetate, then wash with salt water, and extract the aqueous phase with ethyl acetate. The organic layer was collected, dried, concentrated, and column chromatography gave (E)-1-methyl-3-(1`-phenyl-1`-acetoxy)methylene-indol-2-one, yellow solid , melting point 171.1-172.6 °C yield 90%.
[0034] Structure Characterization: 1 H NMR (400MHz) δ: 7.68(d, J=9.6Hz, 2H), 7.55(d, J=7.6Hz, 1H), 7.45-7.43(m, 3H), 7.30(t, J=8.0Hz, 1H ), 7.05(t, J=7.6Hz, 1H), 6.82(d, J=7.6Hz, 1H), 3.19(s, 3H), 2.37(s, 3H); 13 C NMR (100MHz) δ: 167.6, 166.1, 156.9...
Embodiment 2
[0054] Take the preparation of (E)-1-methyl-3-(1`-phenyl-1`-(4-nitrobenzoyloxy))methylene-indol-2-one as an example:
[0055] The compound N-methyl-N-phenyl-phenylpropanamide (0.047g), Pd(OAc) 2 (0.0045g),
[0056] PhI(OAc) 2 (0.0773g), 4-nitrobenzoic acid (0.167g) was placed in the round bottom flask of 25mL, then inject CH 3 CN (3mL), react in a sealed flask at a temperature of 60°C-100°C, but it is best to react at a temperature of 80°C. After the reaction (usually about 2-3 hours), add ethyl acetate, then wash with salt water, water The phase was extracted with ethyl acetate. The organic layer was collected, dried, concentrated, and column chromatography gave (E)-1-methyl-3-(1`-phenyl-1`-(4-nitrobenzoyloxy))methylene-ind Indol-2-one, yellow solid, melting point 172.7-173.1°C, yield 90%.
[0057] Structure Characterization: 1 H NMR (400MHz) δ: 8.42(s, 4H), 7.75(d, J=8.0Hz, 2H), 7.48-7.39(m, 4H), 7.29-7.26(m, 3H), 6.92(t, J= 7.2Hz, 1), 6.84(d, J=8.0Hz, 1H), 3.23(s, 3H...
Embodiment 3
[0071] Take the preparation of (E)-1-methyl-3-(1`-phenyl-1`-phthalimido)methylene-indol-2-one as an example:
[0072] N methyl N phenyl phenylpropanamide (0.047g), phthalimide (0.0882g), Pd(OAc) 2 (0.0045g), PhI(OAc) 2 (0.1288g) was placed in a 25mL round-bottomed flask with polyvinyl fluoride seal, then injected with DCE (3mL), sealed the flask, and reacted at a temperature of 60°C-100°C, but preferably at a temperature of 80°C After 3-10 hours, add ethyl acetate after the reaction is completed, then wash with salt water, extract the aqueous phase with ethyl acetate, collect the organic layer, dry, concentrate, and column chromatography to obtain (E)-1-methyl-3- (1`-phenyl-1`-phthalimido)methylene-indol-2-one, yield 61%, yellow solid, melting point: 202.3204.6°C.
[0073] Structure Characterization: 1 H NMR (400MHz) δ: 7.98-7.96(m, 2H), 7.96-7.84(m, 2H), 7.58(d, J=8.0Hz, 2H), 7.44-7.41(m, 3H), 7.25(t, J=7.6Hz, 1H), 6.99(d, J=7.6Hz, 1H), 6.85-6.80(m, 2H), 3.21(s, 3H); 13 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com