Microporous molecular separation membrane with high hydrothermal stability
A microporous, organic technology, applied in the field of microporous organic-inorganic hybrid membranes, which can solve the problems of inability to provide thermal stability and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1: Preparation of hybrid organic / inorganic silica sol
[0101] The precursor BTESE (1,2-bis(triethoxysilyl)ethane, 96% pure, Aldrich) was distilled to remove impurities and water before use. MTES (methyl-triethoxysilyl-ethane, 99% pure, Aldrich) was used directly. Dry the ethanol before using it, and use sodium aluminum silicate molecular sieve balls with a pore size of 1.0 nm. The precursors were dissolved separately in ethanol. Add MTES / ethanol (molar ratio 1:20) to BTESE / ethanol (molar ratio 1:20).
[0102] In an ice bath, the reaction mixture was stirred using a magnetic stirrer. Water and acid solution (HNO 3 , 65wt%, Aldrich) mixed. Half of the acid / water mixture was added to the precursor mixture and the sol was refluxed at 60°C for 1.5 hours. Next, the remaining half of the acid / water mixture was added and refluxed for an additional 1.5 hours. The reaction was quenched by cooling the reaction mixture while stirring in an ice bath.
[0103] The mo...
Embodiment 2
[0104] Example 2: Preparation of a hydrophobic silica membrane supported by alumina
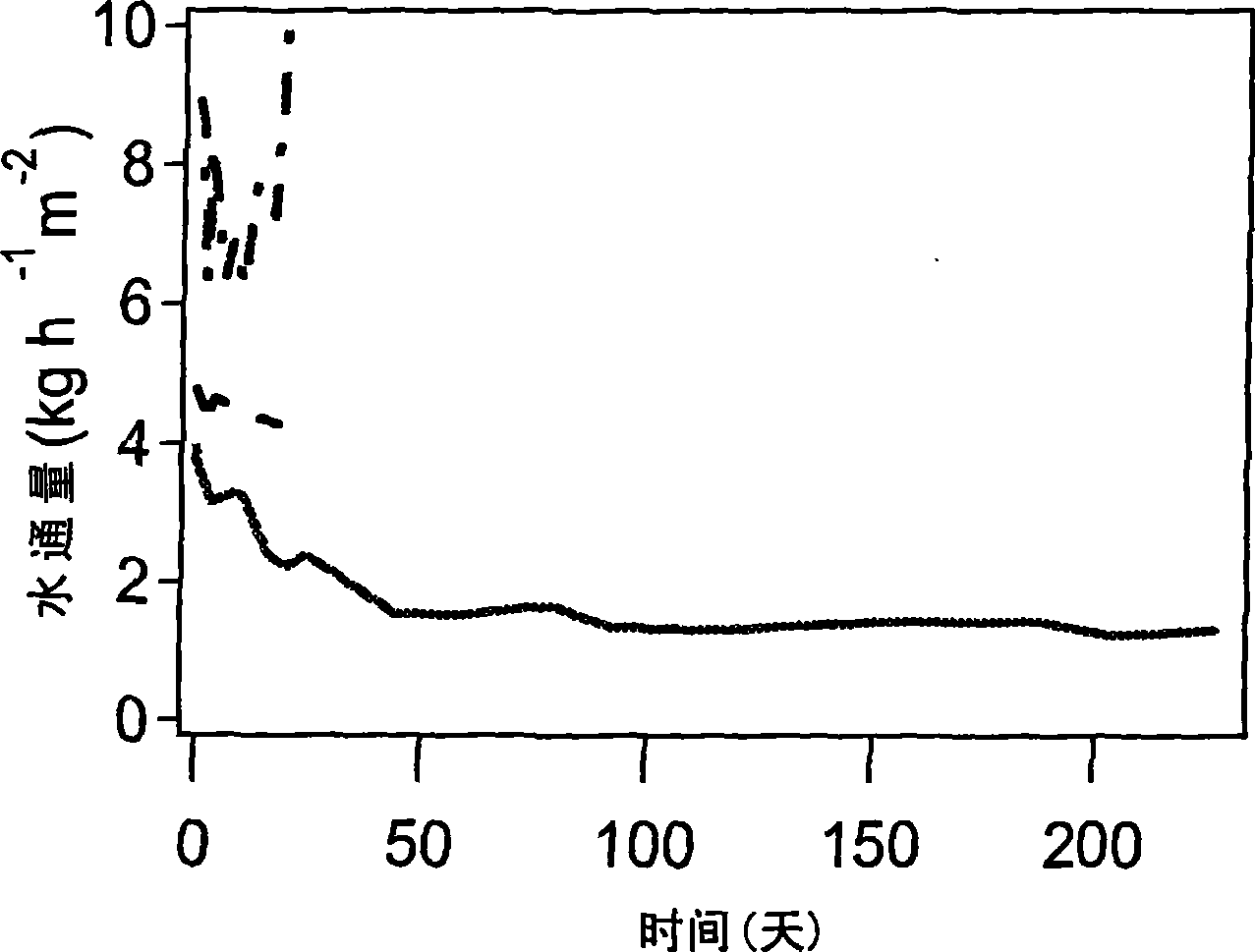
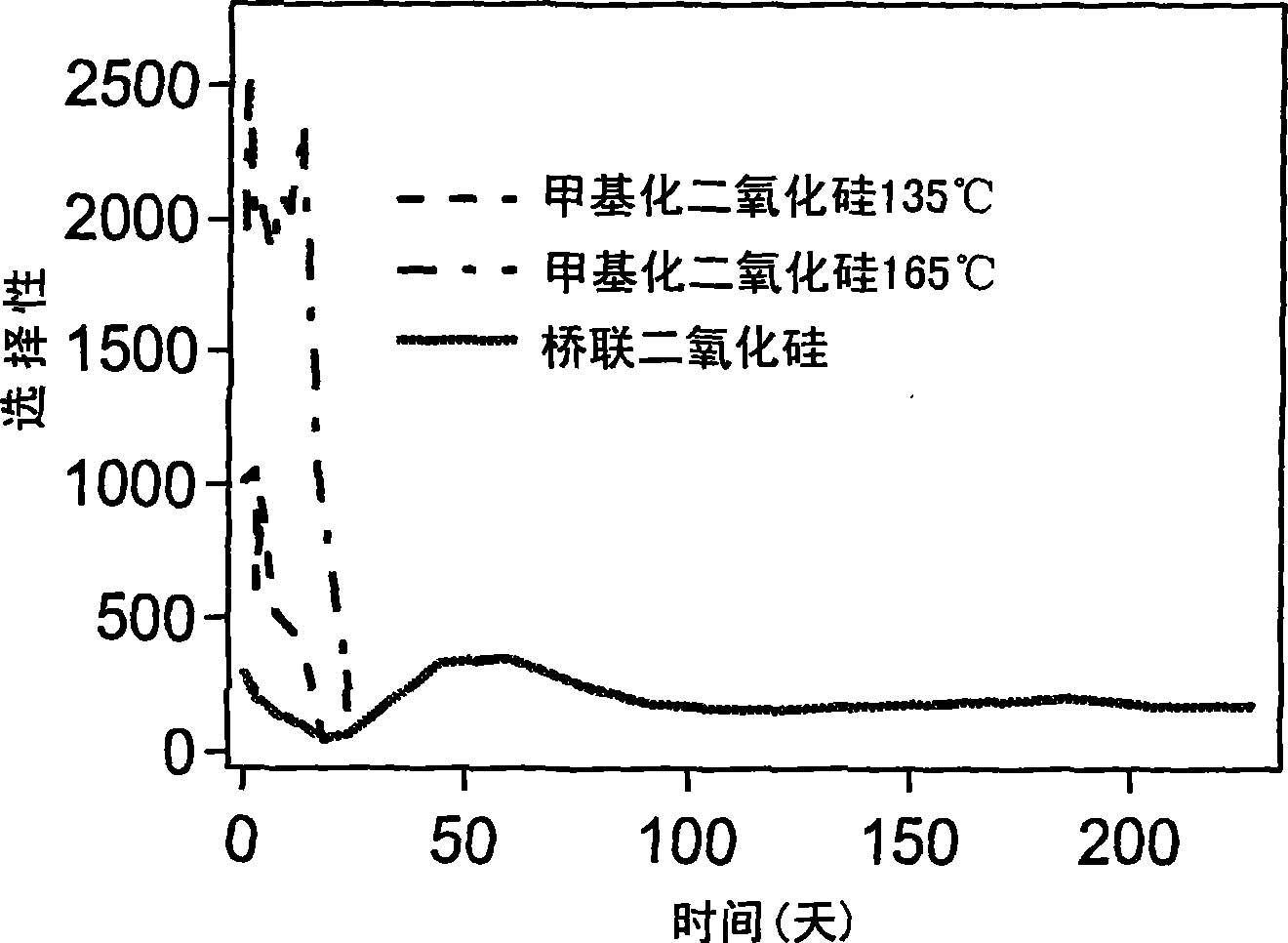
[0105] The gamma-alumina film was dip-coated with the sol prepared according to Example 1. The [BTESE] / [MTES] ratio of the sol is 1, [H 2 O] / ([BTESE]+[MTES]) ratio was 2 (sol A, from membrane A) or 4 (sol B, from membrane B). At 300°C, N 2 In atmosphere, the film was calcined for 3 hours with a heating and cooling rate of 0.5°C / min.
[0106] As described by Campaniello et al. (Chem. 2 In atmosphere, the film was calcined for 3 hours with a heating and cooling rate of 0.5°C / min.
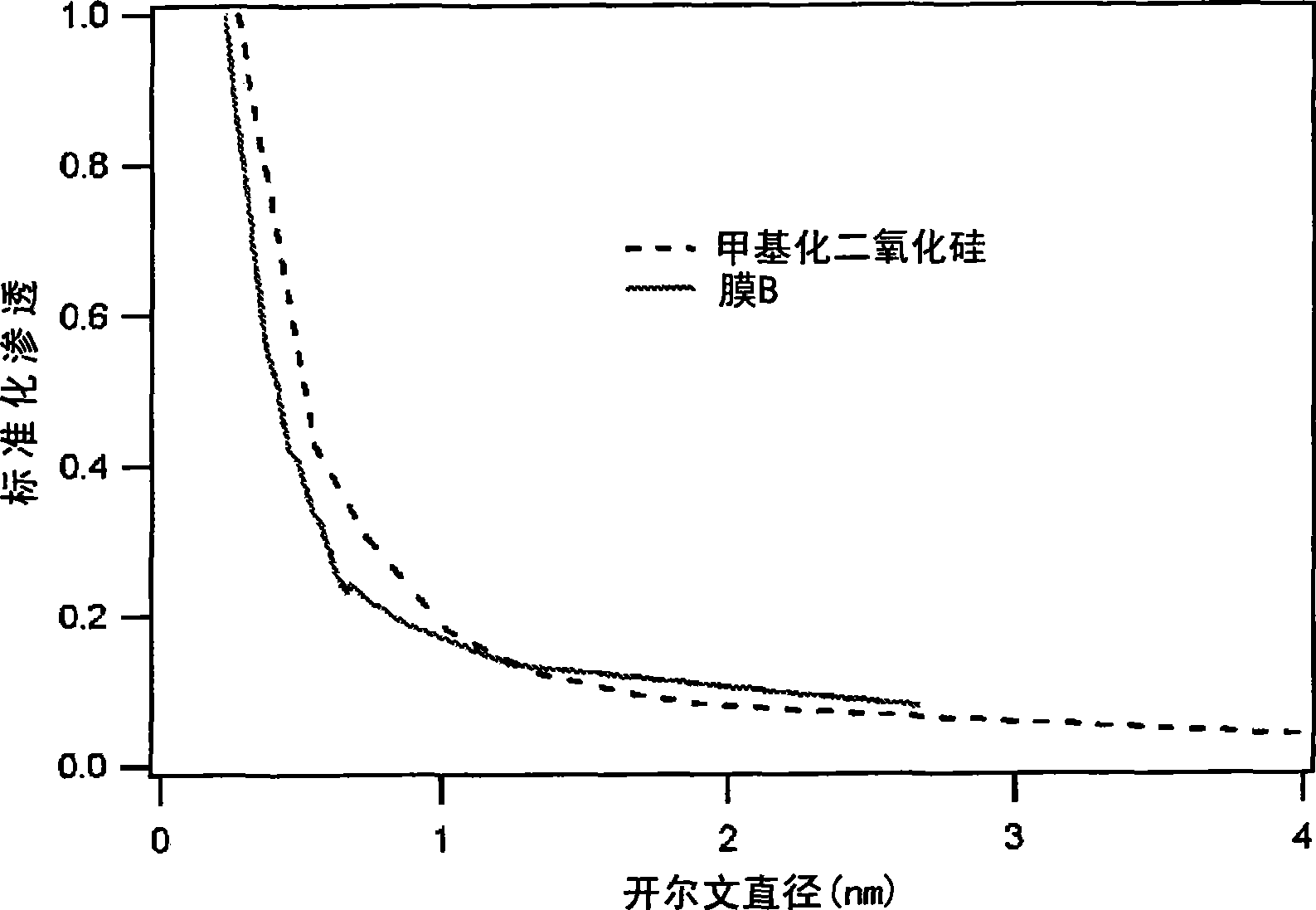
[0107] A microporous layer is thus formed on the tubular membrane with an average pore size of 0.24-0.28 nm as determined by the adsorption technique described above, with substantially no pores larger than 0.30 nm.
[0108] The Kelvin pore size distribution determined by permporometry of this membrane is very similar to that of the methylated silica membrane prepared according to De Vos.
[0109] Pervaporation...
Embodiment 3
[0112] Example 3: Preparation of BTESE-based hybrid organic / inorganic silica sol.
[0113] The precursor BTESE (1,2-bis(triethoxysilyl)ethane, 96% pure, Aldrich) was distilled to remove impurities and water before use. Ethanol (p.a., Aldrich) was used directly. Dissolve the precursor in ethanol. In an ice bath, the reaction mixture was stirred using a magnetic stirrer. Dilute water with acid solution (HNO 3 , 65wt%, Aldrich) mixed. The acid / water / ethanol mixture was added dropwise to the precursor mixture, and the resulting sol was refluxed at 60°C for 2-3 hours. The reaction was quenched by cooling the reaction mixture while stirring in an ice bath.
[0114] The molar ratio of the reactants is [H 2 O] / [BTESE]=(3-6), [H + ] / [BTESE]=(0.02-0.4). The amount of water included with the acid catalyst (HNO 3 ) and water introduced together with the solvent (ethanol).
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com