Use of pharmaceutical composition in preparing medicament for preventing and treating allergic diseases
An allergy, composition technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
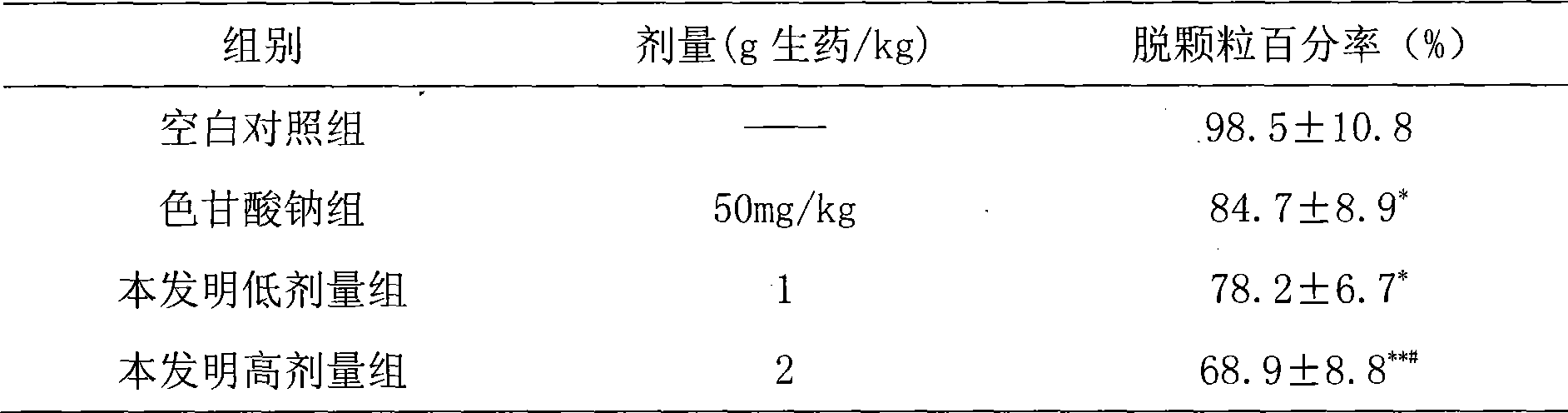
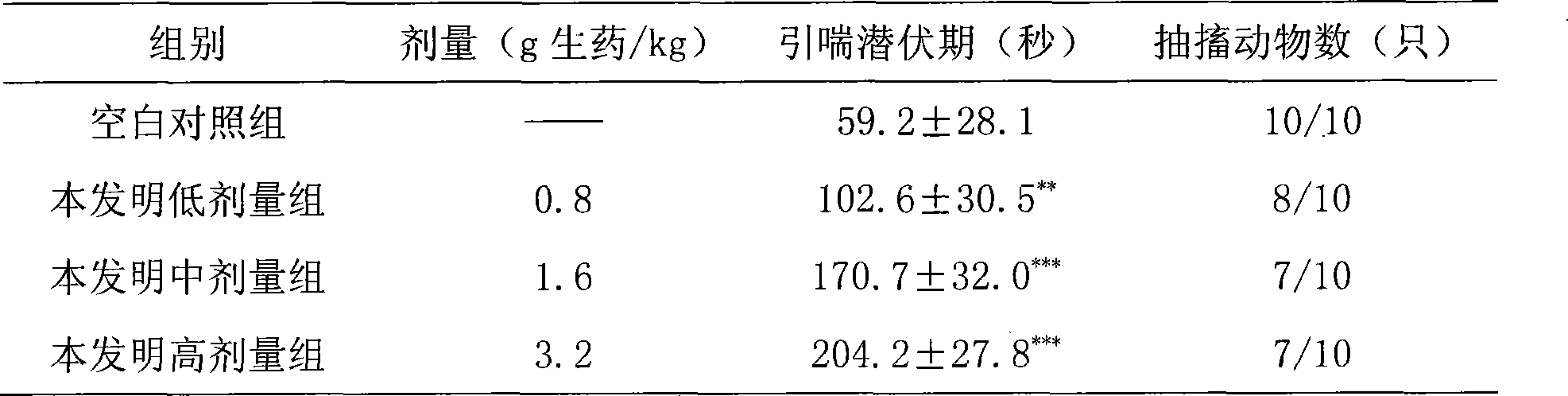
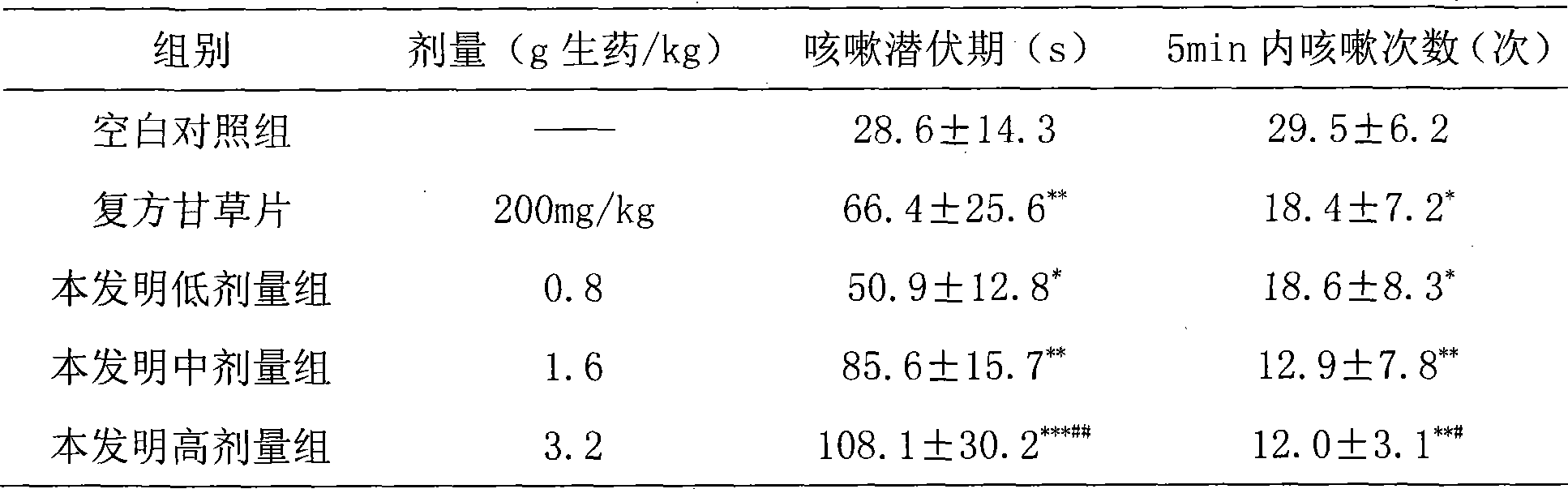
[0137] Take 27g of earthworm and 13g of scorpion for use by superfine grinding; the remaining 66g of astragalus, 27g of red peony root, 27g of salvia miltiorrhiza, 27g of angelica, 27g of chuanxiong, 27g of peach kernel, 13g of safflower, 13g of frankincense, 13g of myrrh, and 20g of milletanus , 27g of Achyranthes bidentata, 20g of cassia twig, 27g of mulberry branch, 27g of leech and other fourteen medicinal materials are ultrafinely ground into fine powder, blended with the above powder, sieved, mixed evenly, add appropriate amount of starch, sodium carboxymethyl cellulose, Stevioside, mixed and passed through 80-mesh sieve, added appropriate amount of water to granulate, dried under reduced pressure at 60°C, granulated, packaged separately, and made into 1000g granules. Usage and dosage: Take 0.8-1.9g once, 3 times a day.
Embodiment 2
[0139] Take 27g of earthworm and 13g of scorpion for use by superfine grinding; the remaining 66g of astragalus, 27g of red peony root, 27g of salvia miltiorrhiza, 27g of angelica, 27g of chuanxiong, 27g of peach kernel, 13g of safflower, 13g of frankincense, 13g of myrrh, and 20g of milletanus , 27g of Achyranthes bidentata, 20g of cassia twig, 27g of mulberry branch, 27g of leech and other fourteen medicinal materials are ultrafinely ground into fine powder, blended with the above powder, sieved, mixed evenly, added starch, mixed evenly, granulated and loaded Capsules, made into 1000g hard capsules. Usage and dosage: Take 0.8-1.6g once, 3 times a day.
Embodiment 3
[0141] Take 27g of earthworm and 13g of scorpion for use by superfine grinding; the remaining 66g of astragalus, 27g of red peony root, 27g of salvia miltiorrhiza, 27g of angelica, 27g of chuanxiong, 27g of peach kernel, 13g of safflower, 13g of frankincense, 13g of myrrh, and 20g of milletanus , Achyranthes bidentata 27g, cassia twig 20g, mulberry branch 27g, leech 27g and other fourteen medicinal materials are ultrafinely pulverized into fine powder, compounded with the above powder, sieved, and mixed. Melt the gelatin, water and glycerin (1:1:0.3-0.45) and keep it warm for later use. Add an appropriate amount of vegetable oil (soybean oil or salad oil) to the medicine powder and mix well. Grind it into a uniform and viscous paste with a colloid mill. Degassing, pressing, and making 1000g soft capsules. Usage and dosage: Take 0.8-1.6g once, 3 times a day.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com