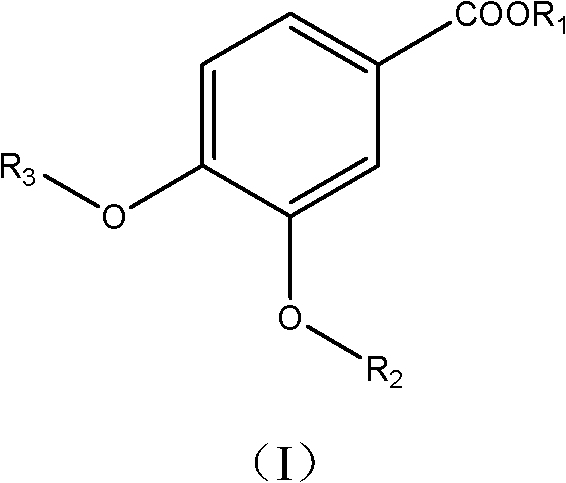
Use of vanillic acid derivatives in the treatment of systemic autoimmune diseases
An autoimmune, vanillic acid technology, applied in nervous system diseases, muscular system diseases, neuromuscular system diseases, etc., can solve problems such as other biological activities that have not been reported, reduce deposition and kidney tissue damage, and inhibit delayed Sexual hypersensitivity, the effect of reducing antibody levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
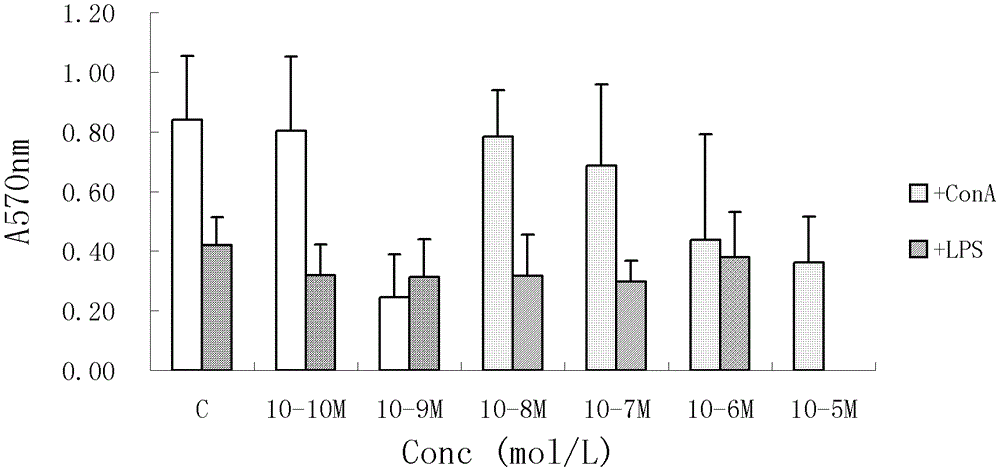
[0026] Example 1. Vanillic acid glucoside has obvious inhibitory effect on the proliferation of mouse spleen T and B lymphocytes cultured in vitro
[0027] 1.1 Materials and methods
[0028] Clean-grade BALB / c mice, male, weighing (19±2) g, were purchased from the Experimental Animal Center of the Chinese Academy of Medical Sciences. LPS, ConA, DMSO, MTT Sigma products. RPMI 1640 complete medium contains penicillin 100U / L, streptomycin 100U / L, glutamine 2mM and 10% fetal bovine serum. The mice were killed by neck dislocation, and the spleen was taken aseptically, and the lymphocyte suspension was prepared according to the routine, and the cells were adjusted to 8×10 cells with RPMI1640 complete medium. 9 / L, 0.1mL / well to inoculate a 96-well plate. Add ConA (final concentration is 2.5mg / L) or LPS (final concentration is 10mg / L), and vanillic acid glucoside (final concentration is 1X10 -9 -1X10 -5 mol / L), 6 replicate wells for each concentration. 37°C, 5% CO 2 cultured i...
Embodiment 2
[0038] Example 2. Vanillic acid glucoside has obvious inhibitory effect on delayed hypersensitivity in mice
[0039] 2.1 Materials and methods
[0040] Balb / c mice, male, 18-20 g, were purchased from the Experimental Animal Center of the Chinese Academy of Medical Sciences. They were randomly divided into three groups, and the experiment started after one week of adaptive feeding. Sensitized mBSA (Sigma) was added with normal saline for injection to 5 mg / mL, and fully emulsified with an equal volume of complete Freund's adjuvant (CFA, Sigma). Except for the normal control group, 100 μl of emulsifier was intradermally injected into the abdomen of each mouse. From the 5th day of drug sensitization, intragastric administration was carried out daily, 0.2ml / bird, for 3 consecutive times. The normal and model control groups were given distilled water, 4 dose groups of test drug vanillic acid glucoside 1.1, 3.3, 10, and 20 mg / Kg body weight, and 2 dose groups of positive drug indo...
Embodiment 3
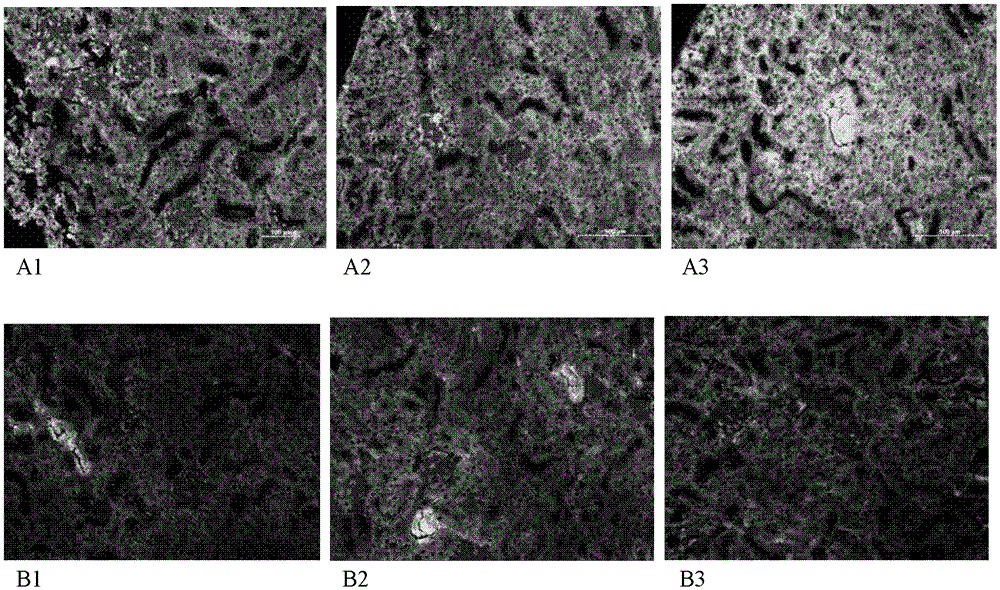
[0047] Example 3. The improvement effect of vanillic acid glucoside on active DNA-induced systemic lupus erythematosus syndrome-like mouse antibody level and renal tissue damage
[0048] 3.1 Materials and methods
[0049] Activation of splenic lymphocytes and extraction of genomic DNA BALB / c mice were sacrificed, spleens were taken aseptically, spleen cell suspensions were routinely prepared, counted with trypan blue (survival rate > 95%), adjusted with RPMI 1640-20% fetal bovine serum Cell 2X10 9 / L, add Con A to make the final concentration 3mg / L, add 20mL per bottle into a culture bottle, culture upright in a 5% CO2 incubator at 37°C, take it out after 48h, and collect activated cells by centrifugation. Active DNA was prepared according to the operation steps of the kit by using a large number of rapid extraction kits for animal genome DNA (Beijing Biotech Biogene Technology Co., Ltd.). Establishment of systemic lupus erythematosus syndrome-like mouse model BALB / c mice, 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com