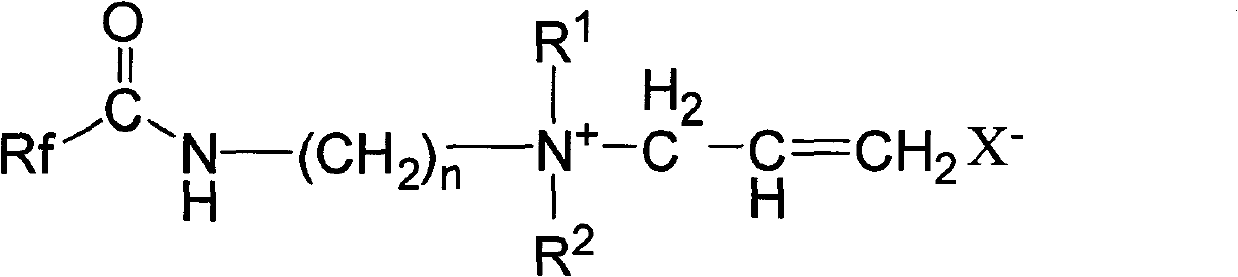
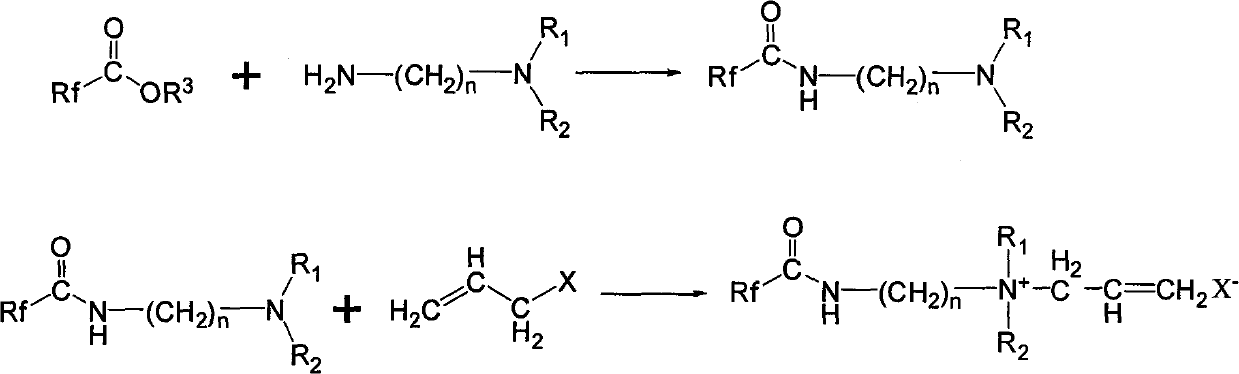
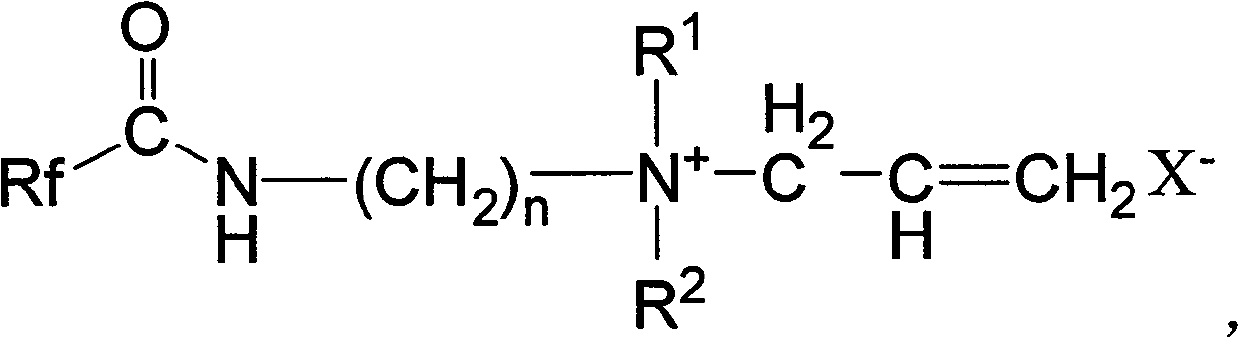
Hexafluoropropylene-based quaternary ammonium salt cationic surfactant, synthesizing method and use
A technology of surfactant and base quaternary ammonium salt, which is applied in the field of perfluoropropenyl quaternary ammonium salt cationic surfactant and its synthesis, can solve the problems of limiting cationic surfactant and the like, achieves good water solubility, simple synthesis method, The effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] Will C 7 f 15 COOCH 3 (42.8g, 0.10mol) and H 2 NCH 2 CH 2 N(C 2 h 5 ) 2 15.6g, 0.12mol) into the reaction flask, heated to 90~120°C for reaction under stirring, while the reaction was distilled to remove the methanol generated by the reaction, and the excess ethyldiamine compound H was distilled off under reduced pressure. 2 NCH 2 CH 2 N(C 2 h 5 ) 2 , that is, the corresponding amide C 7 f 15 CONHCH 2 CH 2 N(C 2 h 5 ) 2 Crude. The crude product is distilled under reduced pressure, and the fraction in the range of 140-160°C / 20mmHg is collected to obtain amide C 7 f 15 CONHCH 2 CH 2 N(C 2 h 5 ) 2 The fine product is 46.5g, and the yield is 93.0%.
[0026] The amide C obtained by the above reaction 7 f 15 CONHCH 2 CH 2 N(C 2 h 5 ) 2 Add it into the reaction bottle, keep the reaction temperature between -5°C and 10°C under the condition of ice bath cooling, slowly add 3-bromopropene dropwise under stirring, after the addition, h...
Embodiment 2
[0028]
[0029] Will C 7 f 15 COOCH 3 (42.8g, 0.10mol) and H 2 NCH 2 CH 2 N(CH 3 ) 2 12.5g, 0.12mol) into the reaction flask, heated to 90-120°C for reaction under stirring, and the methanol produced by the reaction was distilled off at the same time, and the excess methyldiamine compound H was distilled off under reduced pressure. 2 NCH 2 CH 2 N(CH 3 ) 2 , that is, the corresponding amide C 7 f 15 CONHCH 2 CH 2 N(CH 3 ) 2 Crude. The crude product is distilled under reduced pressure, and the fraction in the range of 130-155°C / 20mmHg is collected to obtain amide C 7 f 15 CONHCH 2 CH 2 N(CH 3 ) 2 Fine product 50.5g, yield 95.0%.
[0030] The amide C obtained by the above reaction 7 f 15 CONHCH 2 CH 2 N(CH 3 ) 2 Add it into the reaction bottle, keep the reaction temperature between -5°C and 10°C under the condition of ice bath cooling, slowly add 3-chloropropene dropwise under stirring, and heat to 50°C to 100°C to react After 5-8 hours, after rem...
Embodiment 3
[0032]
[0033] Will C 8 f 17 o 2 COOCH 3 (51.0g, 0.10mol) and H 2 NCH 2 CH 2 N(CH 3 ) 2 12.5g, 0.12mol) into the reaction flask, heated to 90-120°C for reaction under stirring, while the reaction was distilled to remove the methanol generated by the reaction, and the excess methyldiamine compound H was distilled off under reduced pressure. 2 NCH 2 CH 2 N(CH 3 ) 2 , that is, the corresponding amide C 7 f 15 CONHCH 2 CH 2 N(CH 3 ) 2 Crude. The crude product is distilled under reduced pressure, and the fraction in the range of 130-170°C / 20mmHg is collected to obtain amide C 8 f 17 o 2 CONHCH 2 CH 2 N(CH 3 ) 2 The refined product is 55.3g, and the yield is 91.0%.
[0034] The amide C obtained by the above reaction 8 f 17 o 2 CONHCH 2 CH 2 N(CH 3 ) 2 Add it into the reaction flask, keep the reaction temperature between -5°C and 10°C under the condition of ice bath cooling, slowly add 3-chloropropene dropwise under stirring, after the addition, he...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com