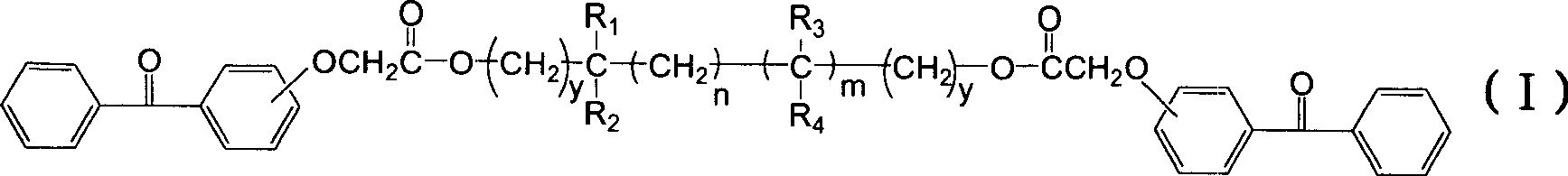
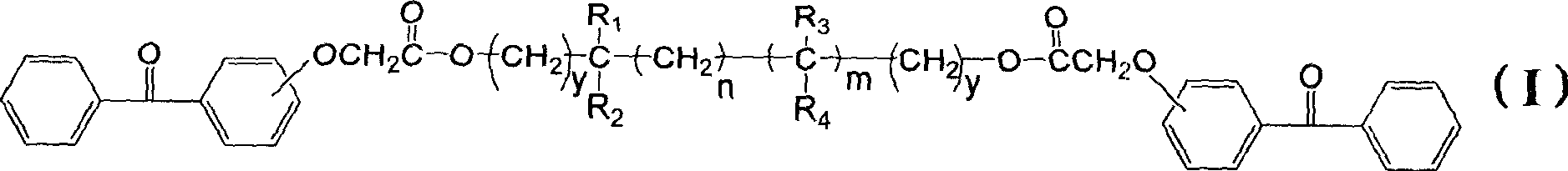
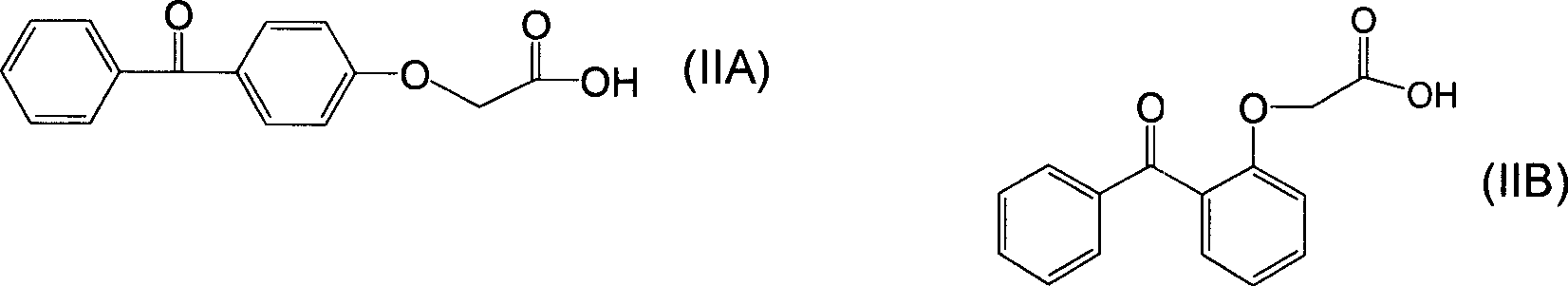Polyfunctional benzophenone derivates and uses as photoinitiators thereof
A technology of benzophenone and compounds, which is applied in the field of benzophenone derivatives, can solve the problems of reduced crosslinking density of cured film and impact on mechanical properties, and achieves the effects of small dissolution tendency, high solubility and low odor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]
[0048] Get a 500ml four-necked flask, put 51.3 grams (0.20mol) of the aforementioned compound of formula (IIA), 10.4 grams (0.10mol) of neopentyl glycol and 1.6 grams of p-toluenesulfonic acid catalyst in 250ml of toluene and carry out azeotropic reflux for 10 hours. Add 100 ml of an aqueous solution containing 2.0 g of sodium bicarbonate to the reaction solution, stir at room temperature for 0.5 hour, separate the water phase, and wash the upper toluene solution twice with 200 ml of deionized water. Then the solution was refluxed and the water was separated with a water separator, and the toluene was removed on a rotary evaporator to obtain 55.4 g of the compound of formula (I-1A) in the form of a light yellow viscous substance, with a yield of 95.4%.
[0049] 1 HNMR (δ, ppm): 0.92 (s, 6H, CH 3 ), 3.96(s, 4H, CH 2 ), 4.73 (s, 4H, OCH 2 ), 6.95 (d, Ar-), 7.46 (t, Ar-), 7.57 (t, Ar-), 7.75 (d, Ar-), 7.83 (d, Ar-).
Embodiment 2
[0051]
[0052] Get a 500ml four-necked bottle, put 51.2 grams (0.2mol) formula (IIA) compound, 16.03 grams (0.1mol) 2,4-diethyl-1,5-pentanediol and 1.6 grams of p-toluenesulfonic acid catalyst Azeotropic reflux was carried out in 120ml of toluene for 10 hours. Add 100 ml of an aqueous solution containing 2.0 g of sodium bicarbonate to the reaction solution, stir at room temperature for 0.5 hour, separate the water phase, and wash the upper toluene solution twice with 200 ml of deionized water. Then the solution was refluxed and the water was separated with a water separator, and the toluene was removed on a rotary evaporator to obtain 61.21 g of the compound of formula (I-2A) in the form of light yellow viscous substance, with a yield of 96.2%.
[0053] 1 HNMR (δ, ppm): 0.86 (m, 6H, CH 3 ), 1.18 (m, 4H, CH 2 ), 1.31 (m, 2H, CH 2 ), 1.68(m, 2H, CH), 4.09-4.136(m, 2H, CH 2 O), 4.72(s, ArOCH 2 ), 6.9 (d, Ar-), 7.47 (t, Ar-), 7.57 (t, Ar-), 7.74 (d, Ar-), 7.81 (d, Ar-). ...
Embodiment 3
[0055]
[0056] Get a 500ml four-necked flask, place 54 grams (0.21mol) of formula (IIA) compound, 11.8 grams (0.1mol) of 3-methyl-1,5-pentanediol and 2 grams of p-toluenesulfonic acid catalyst in 250ml of toluene Azeotropic reflux was carried out for 10 hours. Add 100 ml of an aqueous solution containing 2.0 g of sodium bicarbonate to the reaction solution, stir at room temperature for 0.5 hour, separate the water phase, and wash the upper toluene solution twice with 200 ml of deionized water. Then the solution was refluxed and the water was separated with a water separator, and the toluene was removed on a rotary evaporator to obtain 56.55 g of the compound of formula (I-3A) in the form of a light yellow viscous substance, with a yield of 95.1%.
[0057] 1 HNMR (δ, ppm): 0.928, 0.948 (d, 3H, CH 3 ), 1.485-1.613 (s, H, CH), 1.485-1.746 (m, 4H, 2×C-CH 2 -C), 4.183-4.303(m, 4H, 2×-CH 2 O), 4.70(s, 4H, 2×ArOCH 2 ), 6.9 (d, Ar-), 7.47 (t, Ar-), 7.57 (t, Ar-), 7.74 (d, Ar-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com