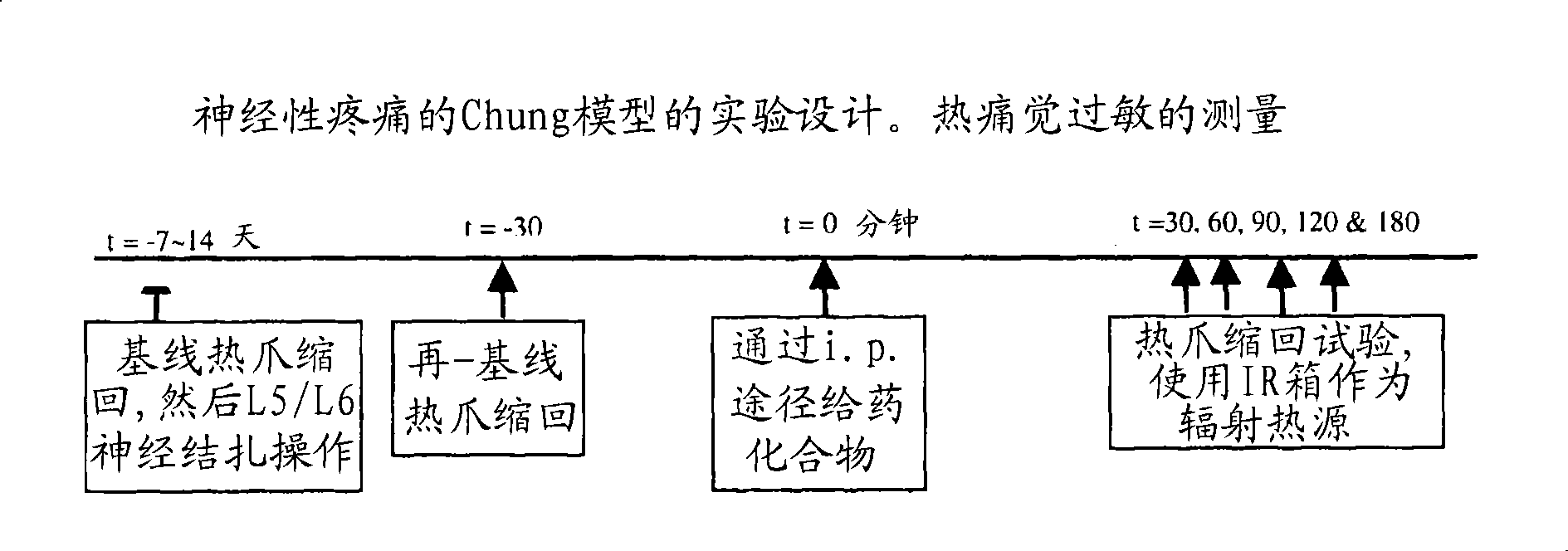
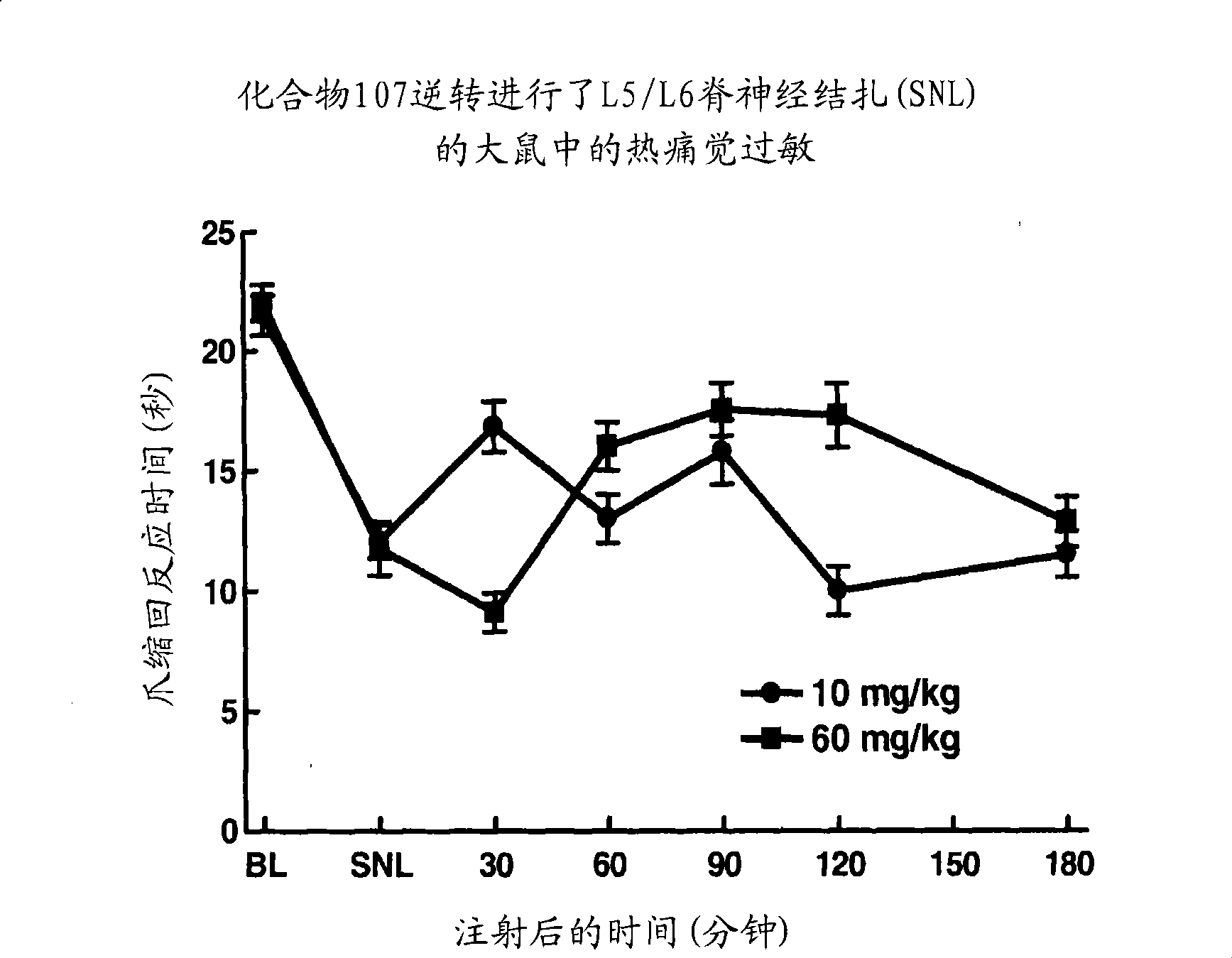
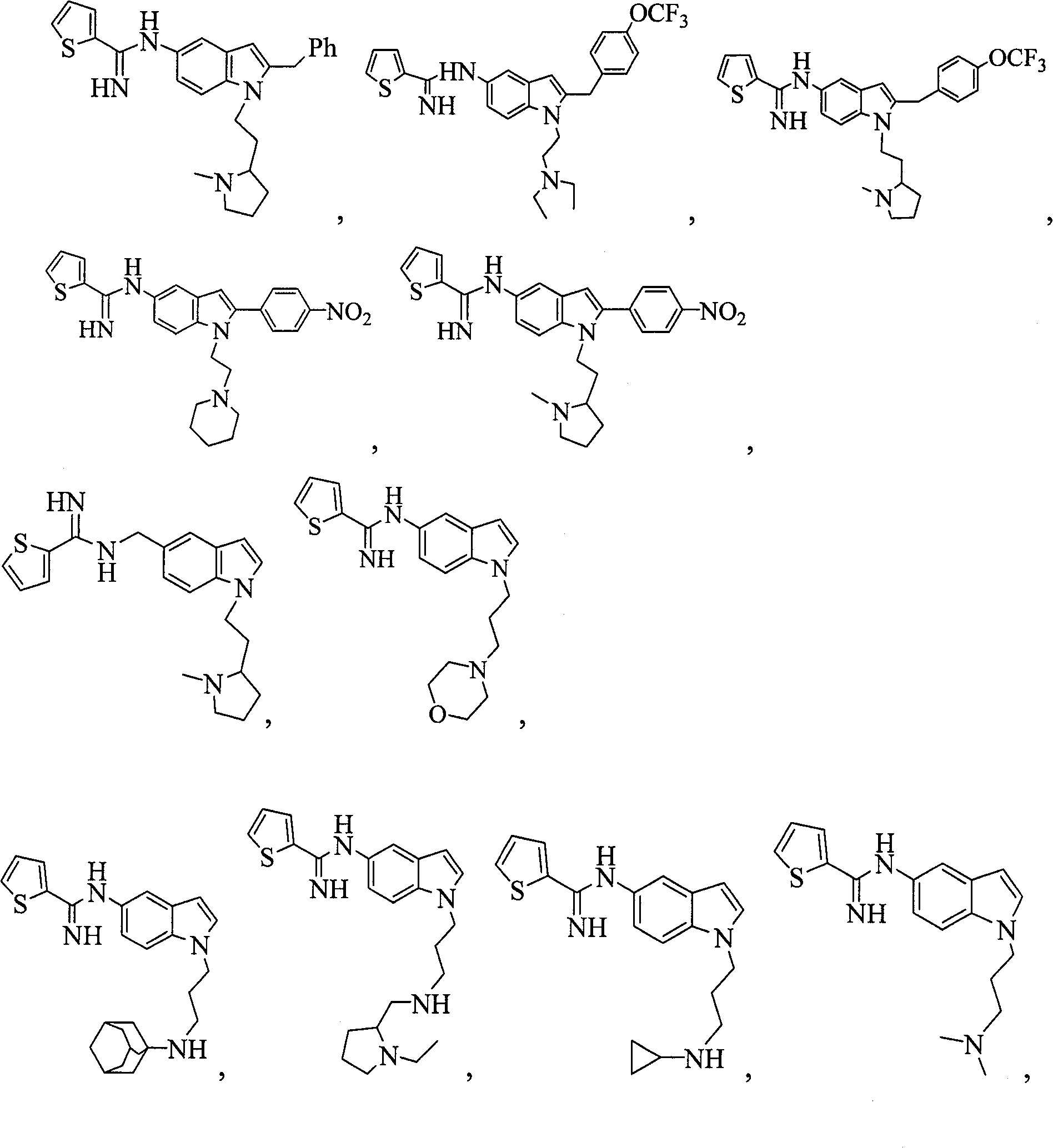
1,5 and 3,6- substituted indole compounds having NOS inhibitory activity
A technology of compounds and heterocyclic compounds, applied in the fields of organic active ingredients, active ingredients of heterocyclic compounds, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0264] Example 1. Preparation of N-(2-benzyl-1-(2-(1-methylpyrrolidin-2-yl)ethyl)-1H-indol-5-yl) Thiophene-2-carboximide (6)
[0265]
[0266] 1-Benzyl-5-nitro-1H-indole (2): Compound 1 (1.0 g, 6.167 mmol) was carried out according to the conditions described in Organic Syntheses, Coll. Vol. 6, p104. The crude product was slurried in boiling hexane, filtered and dried to obtain compound 2.
[0267] 1 H NMR(CDCl 3 )δ 8.61 (d, 1H, J = 2.1 Hz), 8.09 (dd, 1H, J = 2.2, 9.0 Hz), 7.37-7.27 (m, 5H), 7.14-7.09 (m, 2H), 6.74 (d, 1H, J = 3.2 Hz), 5.37 (s, 2H); ESI-MS (m / z, %): 253 (M+1, 100%).
[0268] 2-Benzyl-5-nitro-1H-indole (3): A solution of compound 2 (0.5 g, 1.982 mmol) was treated with polyphosphoric acid as described in Synthetic Communications 1997, 27(12), 2033-2039. The crude product was purified by silica gel column chromatography (EtOAc:hexane, 1:4) to obtain compound 3 (173 mg, 34.6%);
[0269] 1 H NMR(CDCl 3 )δ 8.50 (d, 1H, J = 2.1 Hz), 8.13 (brs, 1H), 8.05 (dd, 1H, J = 2.2,...
Embodiment 2
[0274] Example 2. Preparation of N-(1-(2-(diethylamino)ethyl)-2-(4-(trifluoromethoxy)benzyl)-1H-indole -5-yl)thiophene-2-carboximide (11)
[0275]
[0276] 5-nitro-1-(4-(trifluoromethoxy)benzyl)-1H-indole (7): Add freshly crushed potassium hydroxide (0.692g, 12.332mmol) to a magnetic stir bar And dimethyl sulfoxide (10 mL) in a small argon-purged flask, then the mixture was stirred vigorously for 5 minutes. Compound 1 (500 mg, 3.083 mmol) was added in one portion, and the resulting mixture was stirred at room temperature for 45 minutes, and then briefly cooled to 0°C. 1-(Bromomethyl)-4-(trifluoromethoxy)benzene (1.572g, 6.167mmol) was added dropwise, and then the mixture was stirred for 15 minutes, and then H 2 O dilution. The solution was diluted with ether, transferred to a separatory funnel, and then the organic layer was collected. The aqueous layer was further extracted with ether, and the combined organic layer was extracted with H 2 O (twice), washed with brine, dried ...
Embodiment 3
[0284] Example 3. Preparation of N-(1-(2-(1-methylpyrrolidin-2-yl)ethyl)-2-(4-(trifluoromethoxy)benzyl (Yl)-1H-indol-5-yl)thiophene-2-carboximide (14)
[0285]
[0286] 1-(2-(1-Methylpyrrolidin-2-yl)ethyl)-5-nitro-2-(4-(trifluoromethoxy)benzyl)-1H-indole (12): Compound 8 (75mg, 0.223mmol), 2-(2-chloroethyl)-1-methylpyrrolidine hydrochloride (45.2mg, 0.245mmol), potassium carbonate (92.4mg, 0.669mmol) and anhydrous two Methylformamide (5 mL) was added to a small, argon-purged flask equipped with a magnetic stir bar, and the resulting solution was heated in an oil bath at 65°C for 20 hours. After cooling to room temperature, use H 2 Dilute O and ethyl acetate, transfer to a separatory funnel, and then collect the organic layer. The aqueous layer was further extracted with ethyl acetate, and the combined organic layer was washed with brine (twice), dried over magnesium sulfate, filtered and concentrated to obtain a crude solid. The crude product was purified by dry silica gel co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com