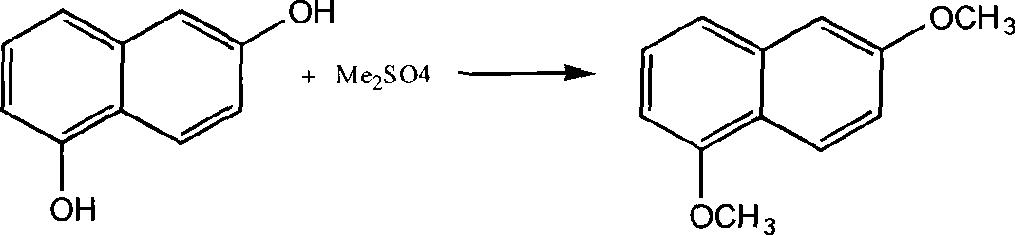
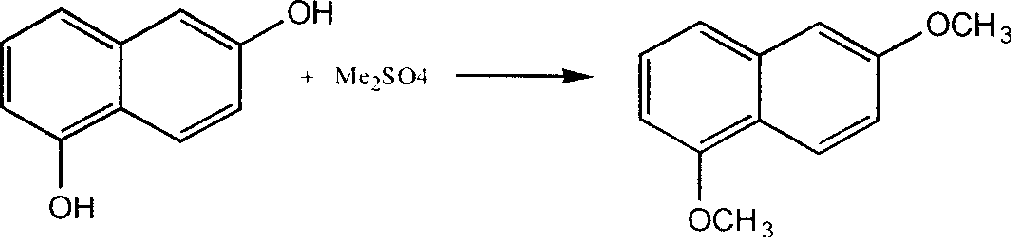Preparation technique of 1,6-dimethoxynaphthalene
A dimethoxynaphthalene, a preparation technology, applied in 1 field, can solve the problems such as difficult recovery of solvent methanol, high cost, expensive acetone, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 20g of 1,6-dihydroxynaphthalene, 102g of petroleum ether (boiling point 60-90°C), 99g of NaOH aqueous solution with a NaOH content of 16%, 1g of hydrosulfite, 0.5g of tetramethylammonium chloride, and drop Add 26g Me 2 SO 4 (15 minutes to finish dripping). Heated to 55-60°C, reacted for 1h. Add 49g NaOH content then and be the NaOH aqueous solution of 16%, dropwise add 20g Me 2 SO 4 (After 15 minutes of dripping), add to 55-60°C, and react for 4 hours.
[0024] Then separate the liquid in a separatory funnel while it is hot, take out the upper oil layer, pour it into a beaker, stir it magnetically, cool it down to 0-5°C in an ice-water bath (keep for 1h), and then filter to obtain yellow crystals of 1,6-dimethoxy naphthalene. Yield 70%, purity 98.6%.
Embodiment 2
[0026] Add 20g 1,6-dihydroxynaphthalene, 150g petroleum ether (boiling point 60-90°C), 115g NaOH aqueous solution with 16% NaOH content, 0.5g hydrosulfite, 1g tetramethylammonium chloride, drop Add 32gMe 2 SO 4 (15 minutes to finish dripping). Heated to 55-60°C, reacted for 0.5h. Add 55g NaOH content then and be the NaOH aqueous solution of 16%, dropwise add 24g Me 2 SO 4 (After 15 minutes of dripping), add to 55-60°C, and react for 7 hours.
[0027] Then separate the liquid in a separatory funnel while it is hot, take out the upper oil layer, pour it into a beaker, stir it magnetically, cool it down to 0-5°C in an ice-water bath (keep for 1h), and then filter to obtain yellow crystals of 1,6-dimethoxy naphthalene. Yield 72%, purity 98.3%.
Embodiment 3
[0029] Operate in the same manner as in Example 2, but replace tetramethylammonium chloride with 0.5g octadecyltrimethylammonium chloride, as a result, yellow crystal 1,6-dimethoxynaphthalene is obtained, yield 71.2%, purity 98.1% %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com