Cinepazide maleate injection and preparation method thereof
A technology of cinepazide maleate and injection, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problem of cinepazide maleate injection. problems such as stability, increased content, and greater impact on drug safety, to achieve the effects of reducing production costs, reducing content, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
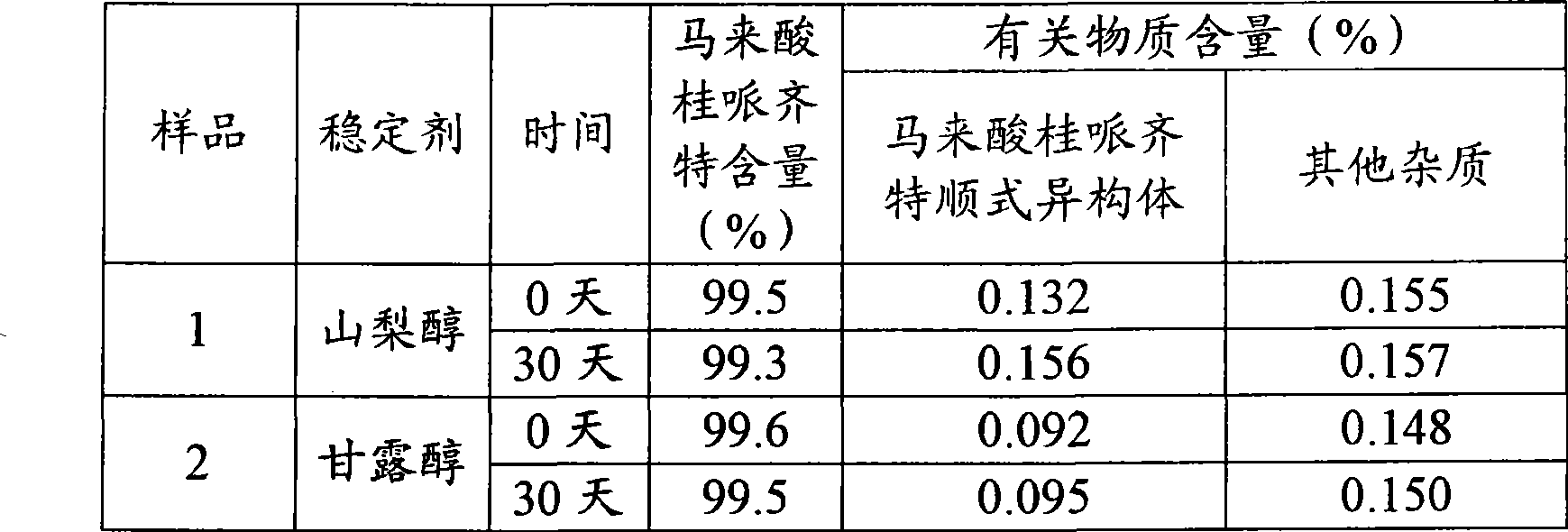
[0025] Sample 1: Operate in dark place, weigh 2.54g of D-sorbitol and add it to 80ml water for injection, stir to dissolve; then weigh 4.05g of cinepazide maleate and add to the above solution, stir to dissolve; use 4% hydrogen phosphate Adjust the pH of the disodium solution to 4.15, and add water for injection to 100ml. A solution of cinepazide maleate was prepared.
[0026] Sample 2: Avoid light, weigh 2.54g of mannitol and add it to 80ml water for injection, stir and dissolve; then weigh 4.08g of cinepazide maleate and add it to the above solution, stir and dissolve; use 4% disodium hydrogen phosphate Adjust the pH of the solution to 4.12, and add water for injection to 100ml. A solution of cinepazide maleate was prepared.
[0027] Table 1 Comparative experiment
[0028]
[0029] As can be seen from the data in Table 1, the content of the two samples has no significant change, and mannitol is selected as the cinepazide maleate cis isomer and other impurities of the s...
preparation example 2
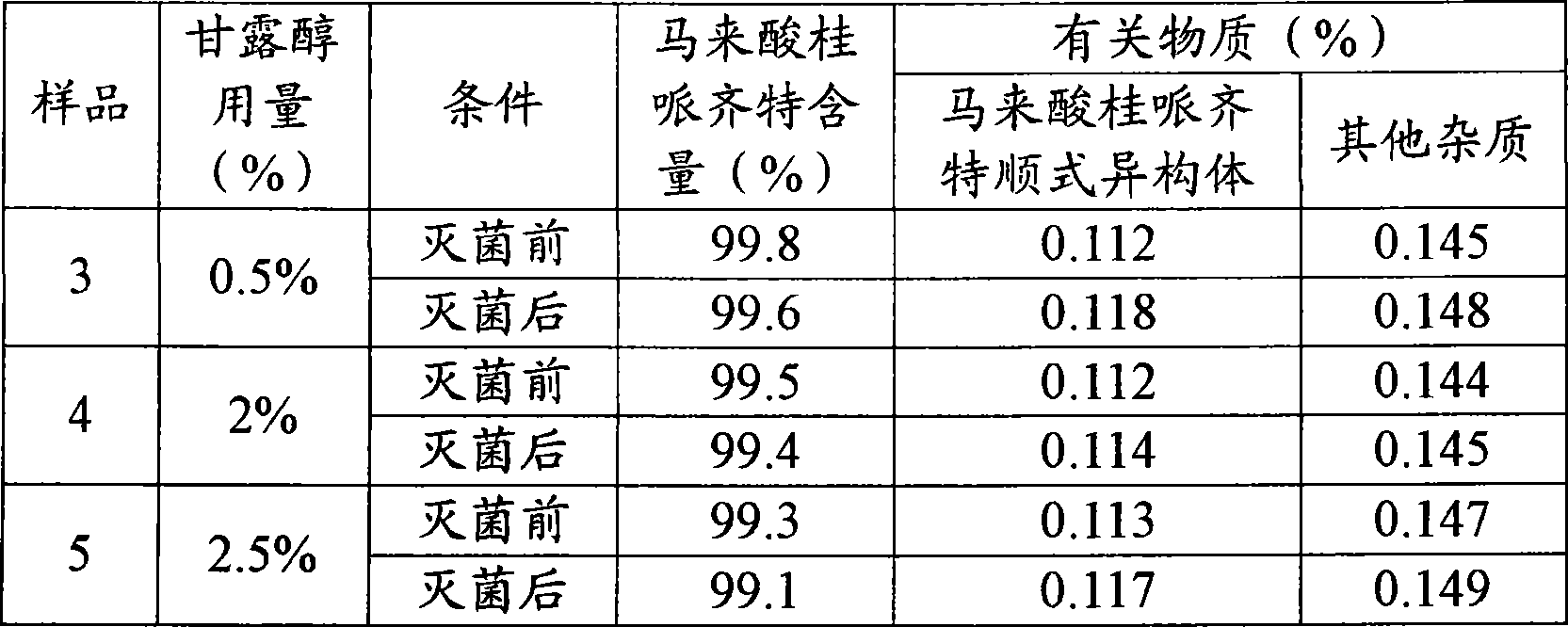
[0033] Sample 3: Weigh 0.25g of mannitol and add it to 40ml of water for injection, stir and dissolve; then weigh 2.03g of cinepazide maleate and add it to the above solution, stir and dissolve; use 4% disodium hydrogen phosphate solution to adjust the pH value When it is 4.05, add water for injection to 50ml, filter, and seal.
[0034] Sample 4: Weigh 1.08g of mannitol and add it to 40ml of water for injection, stir and dissolve; then weigh 2.04g of cinepazide maleate and add it to the above solution, stir and dissolve; use 4% disodium hydrogen phosphate solution to adjust the pH value When it is 4.01, add water for injection to 50ml, filter, and seal.
[0035] Sample 5: Weigh 1.26g of mannitol and add it to 40ml of water for injection, stir and dissolve; then weigh 2.06g of cinepazide maleate and add it to the above solution, stir and dissolve; use 4% disodium hydrogen phosphate solution to adjust the pH value When it is 4.08, add water for injection to 50ml, filter, and se...
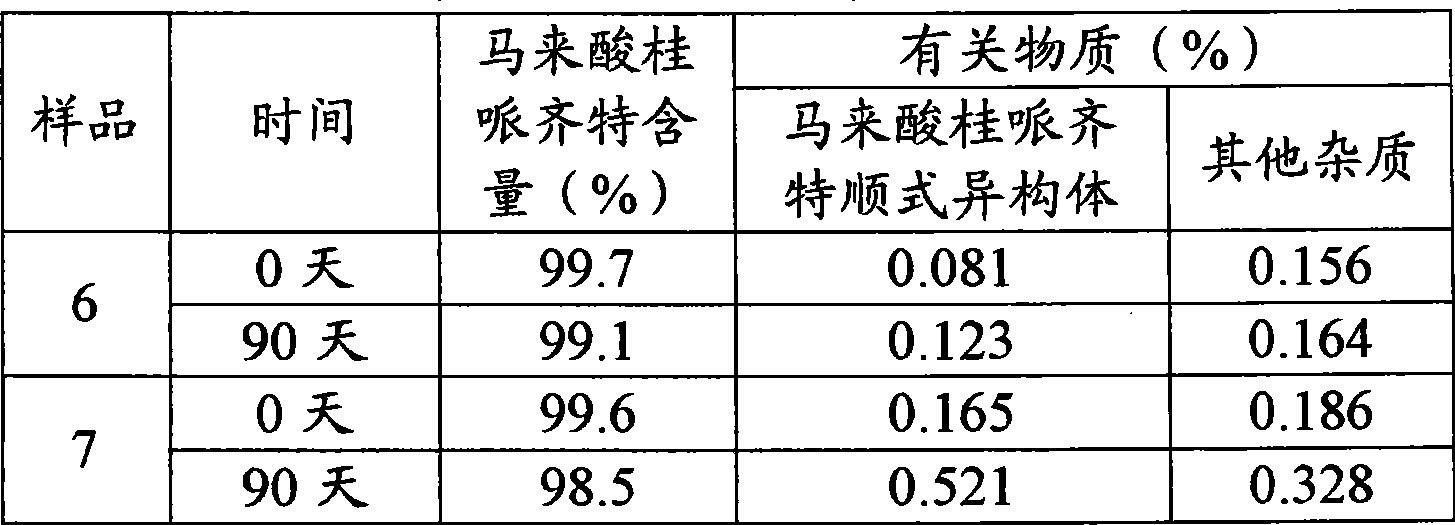
Embodiment 1
[0054] 1. Prescription:
[0055]
[0056] 2. The preparation process, the following steps are all operated in the dark:
[0057] (1) Weigh the prescribed amount of mannitol and add it to 1600ml water for injection, stir to dissolve.
[0058] (2) Add the prescribed amount of cinepazide maleate into the above solution, and stir to dissolve.
[0059] (3) 4% disodium hydrogen phosphate solution to adjust the pH value to 4.15.
[0060] (4) Add water for injection to 2000ml, add 2g of activated carbon, keep stirring at 50°C for 20min, and decarbonize by filtration.
[0061] (5) Intermediate product detection: pH value and content of cinepazide maleate.
[0062] (6) After the intermediate product is qualified, it is filtered with a 0.22 μm microporous membrane, and then potted.
[0063] (7) Sterilize at 121°C for 15 minutes with high temperature and damp heat.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com