Medicine combination capable of resisting fatigue and radiation hazard and preparation method thereof
A technology of composition and medicine, which is applied in the field of pharmaceutical composition with anti-body fatigue and radiation hazards and its preparation, can solve the problem of few products, and achieve the effects of simple process, enhancing body immunity, and good radiation hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
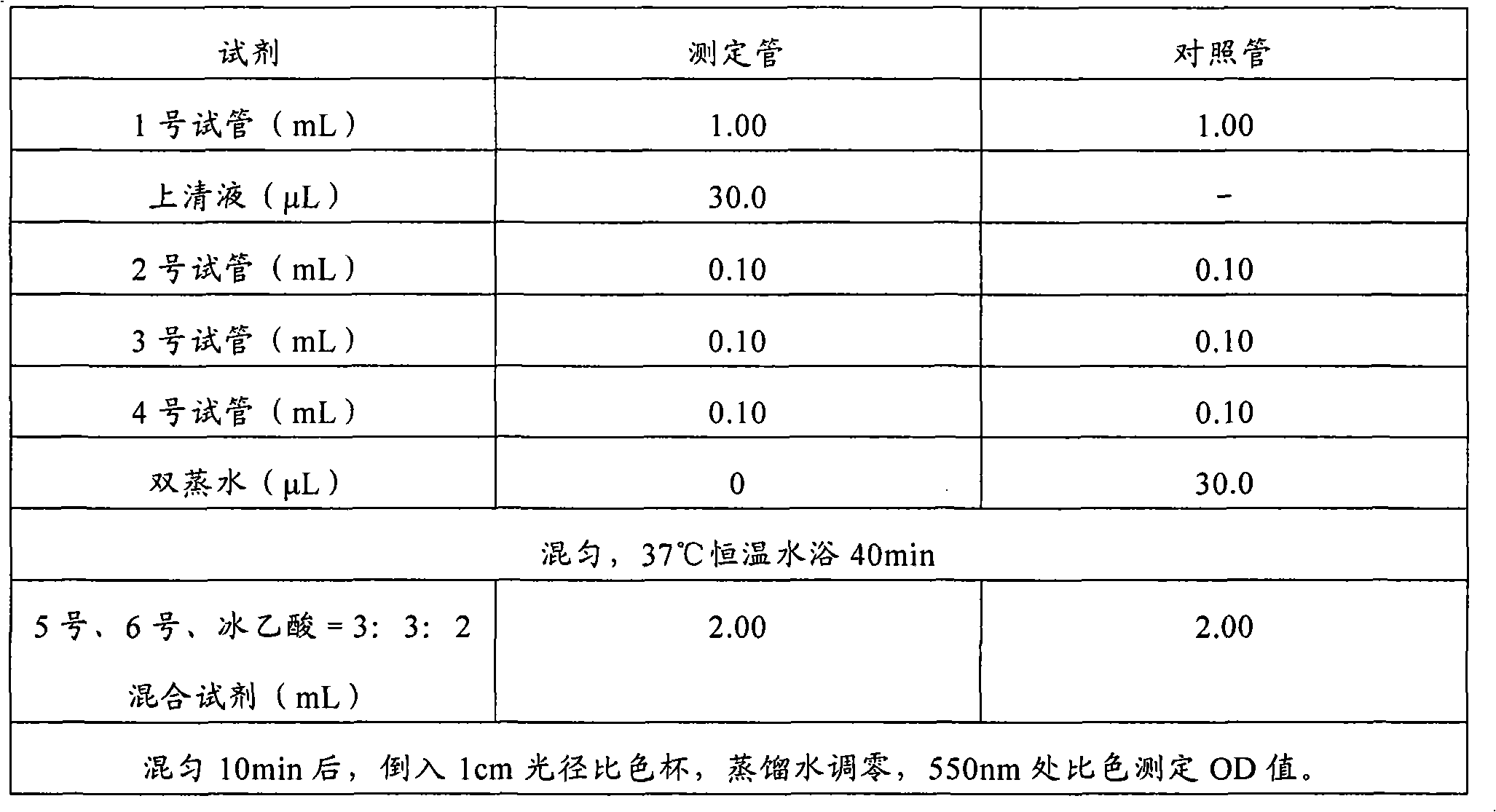
Image
Examples
Embodiment 1
[0058] The preparation of embodiment 1 capsule
[0059] Weigh 750g Rhodiola rosea, 500g Agaricus blazei, 350g Acanthopanax, and 200g Angelica;
[0060] Put Rhodiola rosea, Acanthopanax and Angelica in a multifunctional extraction tank, and extract twice with 70% ethanol under reflux, add 10.5L of 70% ethanol for the first time, reflux for 2 hours, and add 8L of 70% ethanol for the second time , reflux extraction for 1 hour, the extracts were filtered separately, combined, placed in a vacuum concentration pot, and ethanol was recovered under reduced pressure, concentrated to a relative density of about D=1.10-1.20g / ml (50°C), and transported to the vacuum through the pipeline In the concentration tank, concentrate until the relative density is about D = 1.20 ~ 1.30g / ml (50°C), pour it into a stainless steel plate for vacuum drying, and place it in a vacuum drying oven at a vacuum degree of -0.08MPa and a temperature of 80°C Dried to obtain dry extract (moisture content <5%, yi...
Embodiment 2
[0066] The preparation of embodiment 2 tablet
[0067] Weigh 700g Rhodiola rosea, 550g Agaricus blazei, 300g Acanthopanax, and 220g Angelica;
[0068] Rhodiola rosea, Acanthopanax and Angelica were reflux extracted twice with 65% ethanol. For the first time, 9L of 65% ethanol was added, and reflux was extracted for 2 hours. For the second time, 7.5L of 65% ethanol was added, and reflux was extracted for 1 hour. Filter separately, combine, place in a vacuum concentration pot, recover ethanol under reduced pressure, concentrate to a relative density of about D=1.20-1.30g / ml (50°C), and vacuum-dry to obtain a dry extract (moisture <5%, yield About 15~18%);
[0069] Agaricus blazei is decocted twice with water, the first time is decocted with 5.5L water for 2 hours, the second time is decocted with 4.4L water for 1 hour, the decoction is filtered, combined, and concentrated until the relative density is D=1.20~1.30g / ml or so (50°C), vacuum-dried to obtain a dry extract (moistur...
Embodiment 3
[0072] The preparation of embodiment 3 granules
[0073] Weigh 850g of Rhodiola rosea, 600g of Agaricus blazei, 250g of eleuthero, and 150g of angelica;
[0074] Rhodiola rosea, Acanthopanax and Angelica were extracted twice with 80% ethanol under reflux. For the first time, 11L of 80% ethanol was added, and reflux was extracted for 2 hours. For the second time, 9L of 80% ethanol was added, and reflux was extracted for 1 hour. The extracts were respectively Filtrate, combine, place in a vacuum concentration pot, reclaim ethanol under reduced pressure, concentrate to a relative density of about D=1.20~1.30g / ml (50°C), and vacuum dry to obtain a dry extract (moisture content <5%, yield approx. 15~18%);
[0075] Agaricus blazei is decocted twice with water, the first time is decocted with 6L water for 2 hours, the second time is decocted with 5L water for 1 hour, the decoction is filtered, combined, and concentrated until the relative density is D=1.20~1.30g / ml About (50 ℃), va...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com