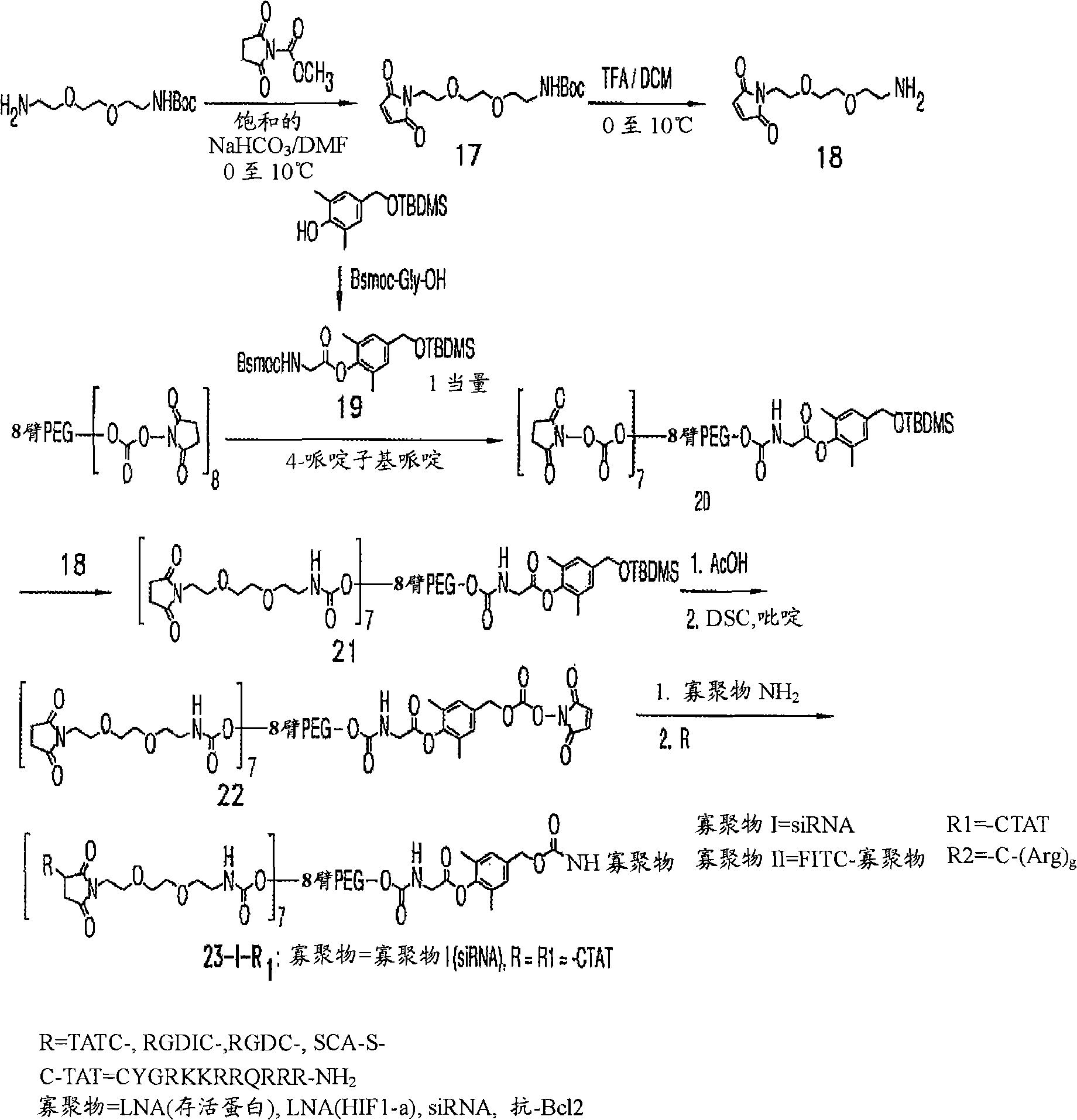Polymeric conjugates containing positively-charged moieties
A technology of polymers and compounds, applied in the directions of medical preparations containing active ingredients, organic active ingredients, ester active ingredients, etc., can solve the problems of unsatisfactory results and achieve the effect of improving cellular uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
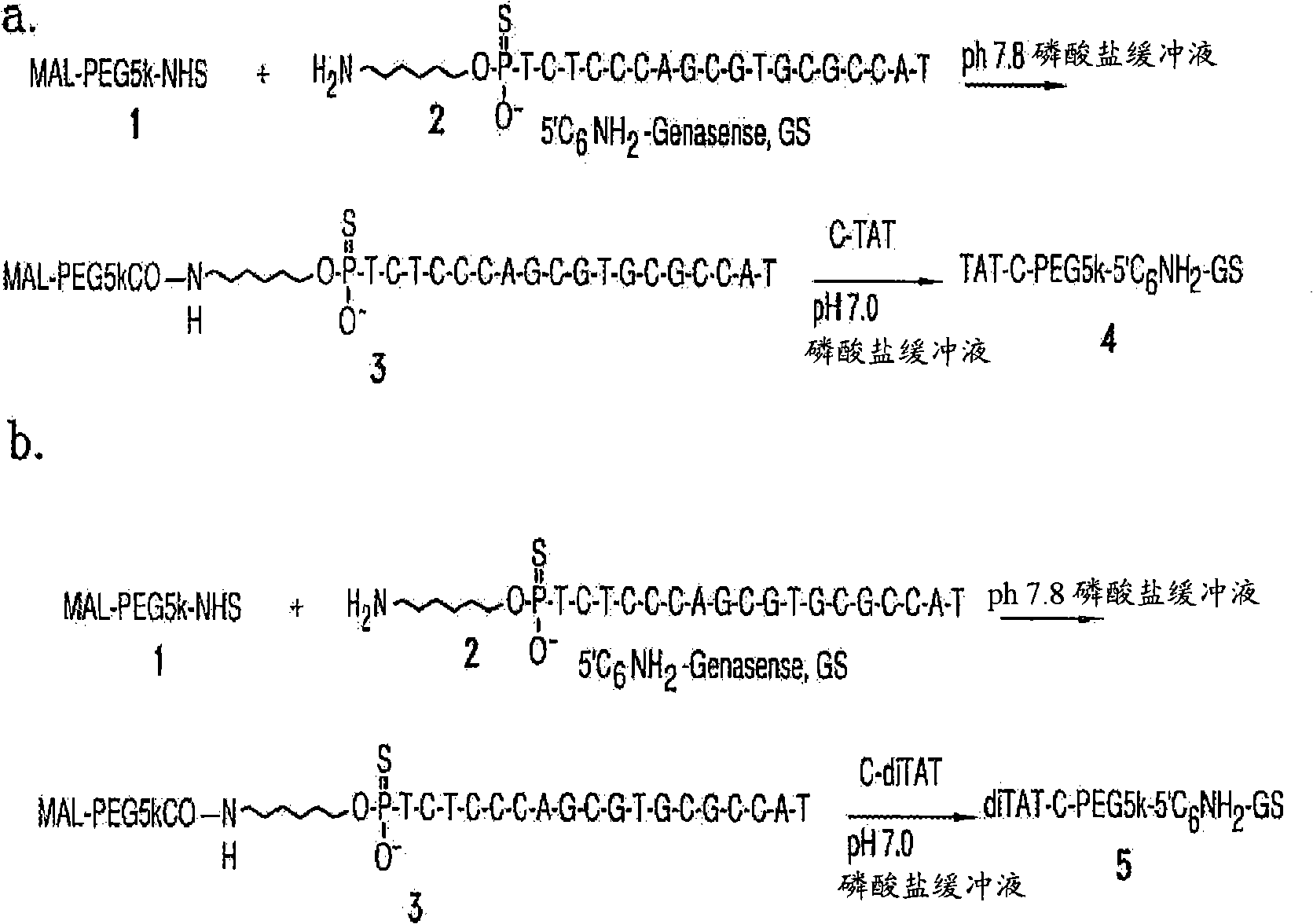
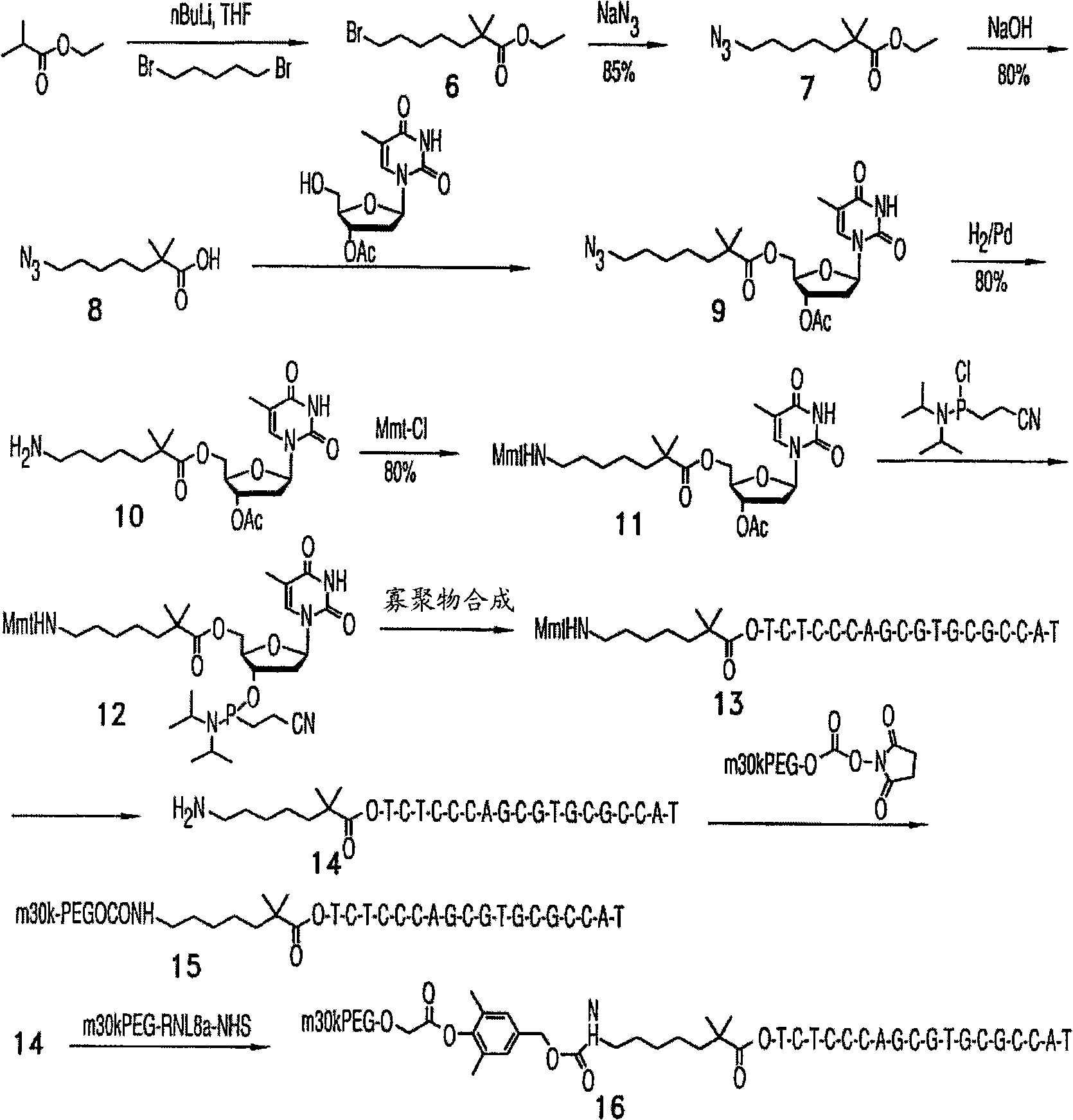
[0248] The oligonucleotide can be, for example, an oligonucleotide having the same or substantially similar nucleotide sequence as Genasense (a / k / a oblimersen sodium (oblimersensodium), produced by Genta Inc., Berkeley Heights, NJ) acid. Genasense is an 18-polyphosphorothioate antisense oligonucleotide, TTCTCCCAGCGTGCGCCAT (SEQ ID NO: 6), which is complementary to the first six codons of the initiation sequence of human bcl-2 mRNA (human bcl-2 mRNA is the basis of this technology known in the art and described, for example, in US Patent 6,414,134 as SEQ ID NO: 19, incorporated herein by reference). The U.S. Food and Drug Administration (FDA) granted Orphan Drug status to Genasense in August 2000. Preferred embodiments include:
[0249] (i) Antisense Survivin LNA (SEQ ID NO: 3)
[0250] m C s -T s - m C s -A s -a s -t s -c s -c s -a s -t s -g s -g s - m C s -A s -G s -c;
[0251] The uppercase letters represent LNA, and "s" represents the phosphorothioate ...
Embodiment 1
[0481] Example 1. Compound 3:
[0482] To a solution of Compound 2 (10 mg, 1.7 μmol) in PBS buffer (5 mL, pH 7.8) was added Mal-PEG5k-NHS (100 mg, 17 μmol) from NOF Corporation and stirred at room temperature for 2 hours. The reaction mixture was diluted with water to 20 mL and loaded onto a Poros HQ, strong anion exchange column (10 mm x 1.5 mm, bed volume ~16 mL) with 20 mM Tris-HCl buffer, pH 7.0 (Buffer A) Pre-equilibrated. The column was washed with 3-4 column volumes of buffer A to remove excess PEG linker. The product was then eluted with a gradient of 0 to 100% 1 M NaCl in 20 mM Tris-HCl buffer (pH 7.0, buffer B) at a flow rate of 10 mL / min for 10 minutes, followed by 100% buffer B 10 minutes. The eluted product was desalted with HiPrep desalting column (50 mL) and lyophilized to obtain compound 3. Yield 6 mg (oligo equivalent, 60%).
Embodiment 2
[0483] Example 2. Compound 4:
[0484] To a solution of compound 3 in PBS buffer (6 mL, pH 7.0), peptide C-Tat (5 mg, 3 μmol) was added and stirred at room temperature for 2 hours. The reaction mixture was diluted to 20 mL with water and loaded onto a Resource S, strong cation exchange column (10 mm x 1.5 mm, column bed volume ~16 mL) with 100 mM K 2 HPO 4 , 5M urea buffer, pH 6.5 (buffer A) pre-equilibrated. The column was washed with 3-4 column volumes of buffer A to remove unreacted PEG-oligocompound. The product was then eluted with a gradient of 0 to 100% 2M KBr (buffer B) for 10 minutes at a flow rate of 10 mL / min, followed by 100% buffer B for 10 minutes. The eluted product was desalted with HiPrep desalting column (50 mL) and lyophilized to obtain compound 4. Yield 2 mg (oligomer equivalent, 30%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com