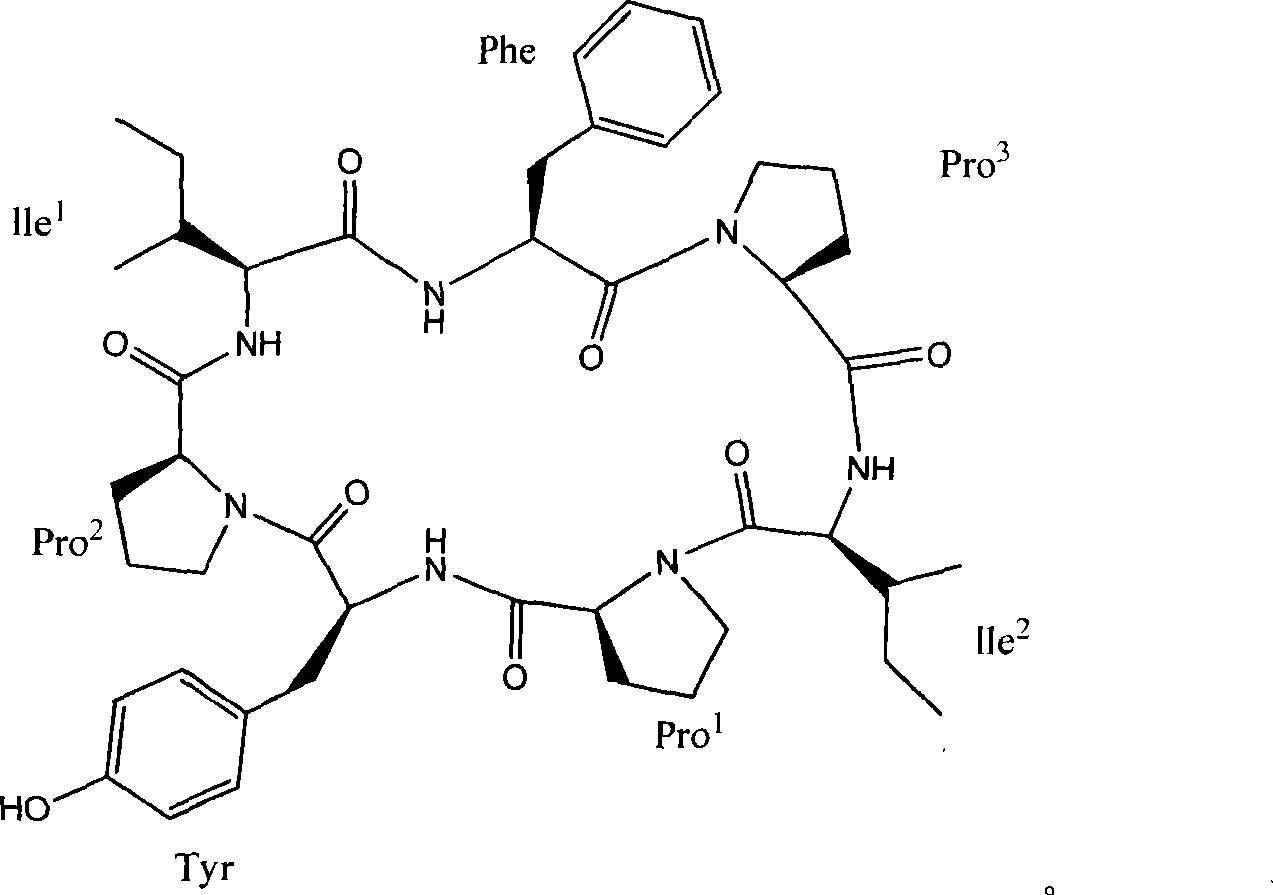
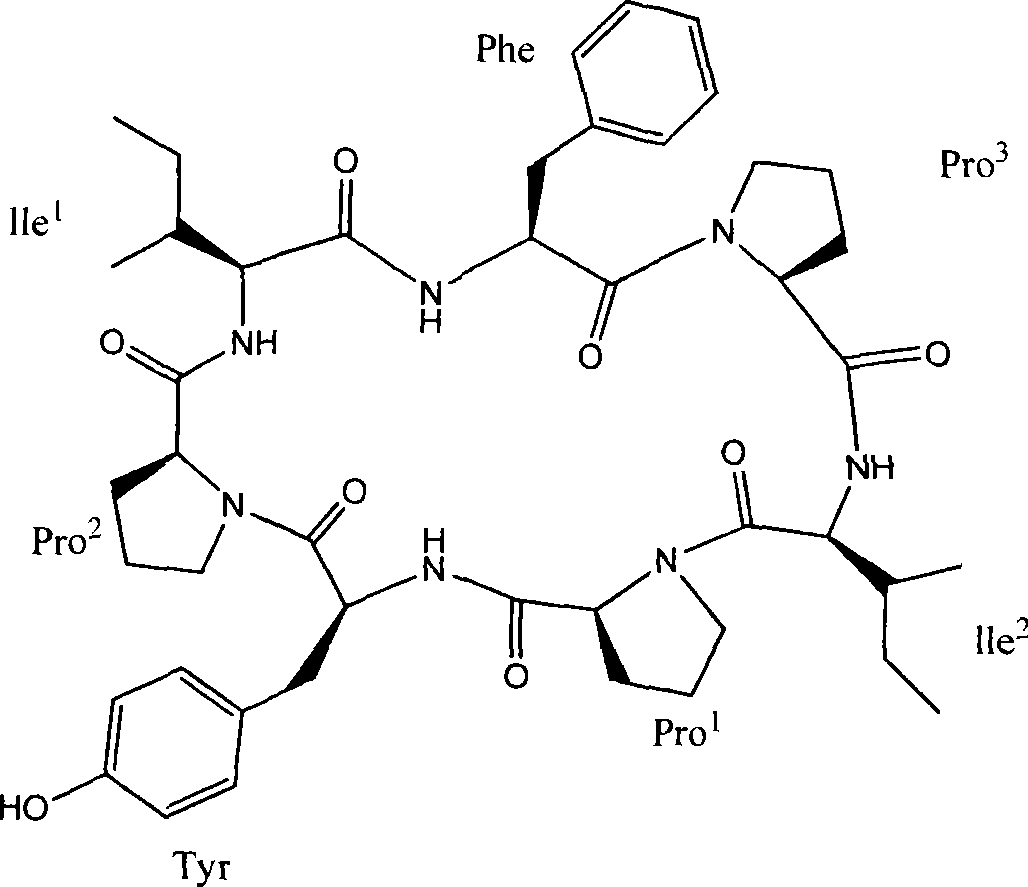
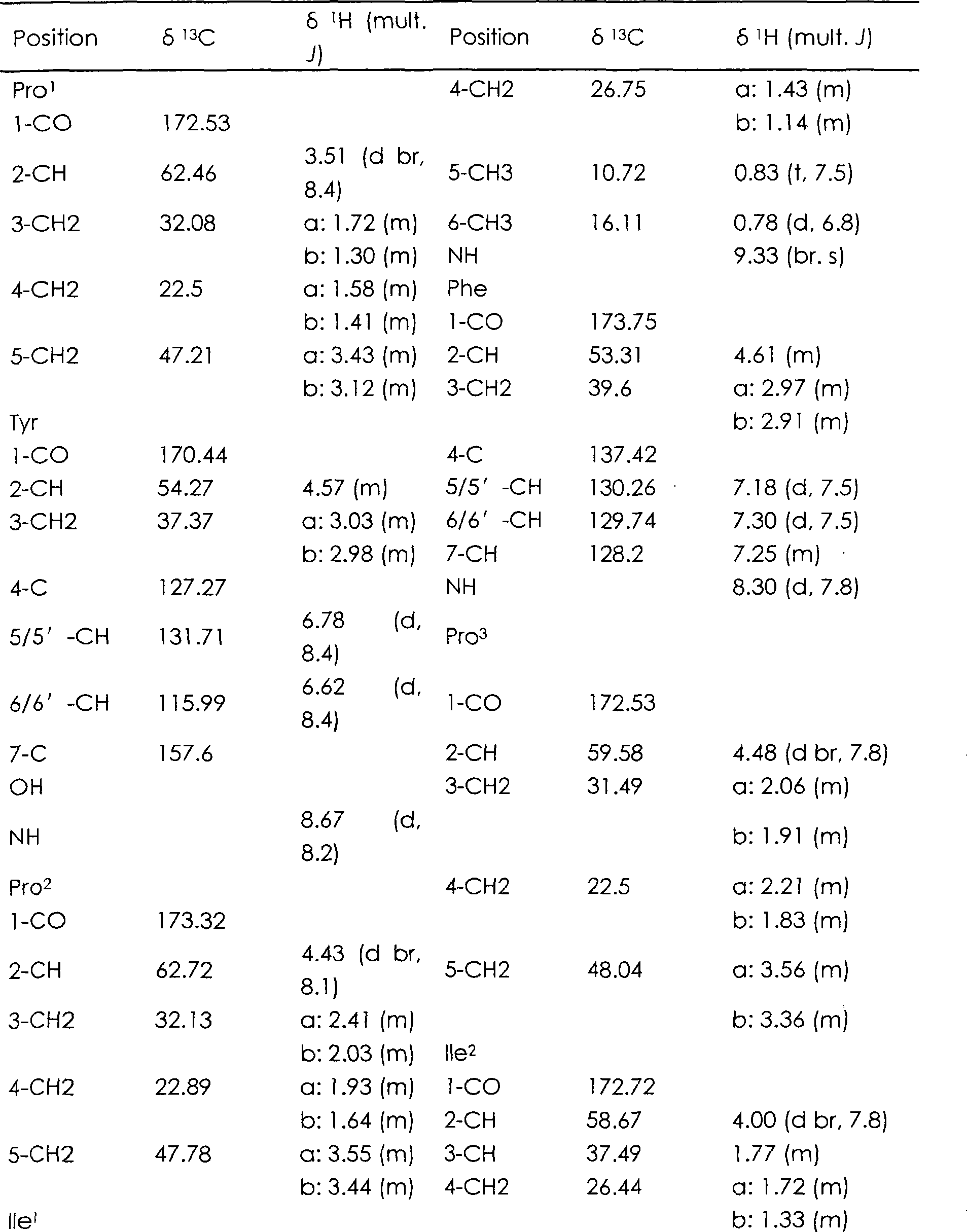
Cyclic heptapeptide compound in Hsisha sponge and application thereof
A compound and cyclic heptapeptide technology, applied in the directions of cyclic peptide components, peptides, drug combinations, etc., can solve the problems of cyclic heptapeptide compounds with no anti-tumor activity, and achieve the effects of significant anti-tumor activity and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1. Preparation of compounds of the present invention
[0015] Take 15 kg of dried and pulverized brown flat sponge, extract by cold soaking in 50 L of 95% ethanol for 8 times, each time for one week, combine the extracts, concentrate the extracts under reduced pressure to obtain extracts, and extract with 50% ethyl acetate aqueous solution, To obtain the total ethyl acetate extract, suspend the total ethyl acetate extract with 90% methanol, degrease with petroleum ether, add water to adjust the methanol concentration of the suspension to 60%, extract 3 times with dichloromethane, and concentrate Extract, get crude extract 55g;
[0016] 55 g of the above-mentioned crude extract was passed through a Sephadex LH-20 gel column, and the 2 Cl 2 -MeOH=1:1 elution, according to the thin layer chromatography detection results, collect the eluate containing cyclic peptide compounds, and then perform reverse phase silica gel column chromatography, use methanol with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com