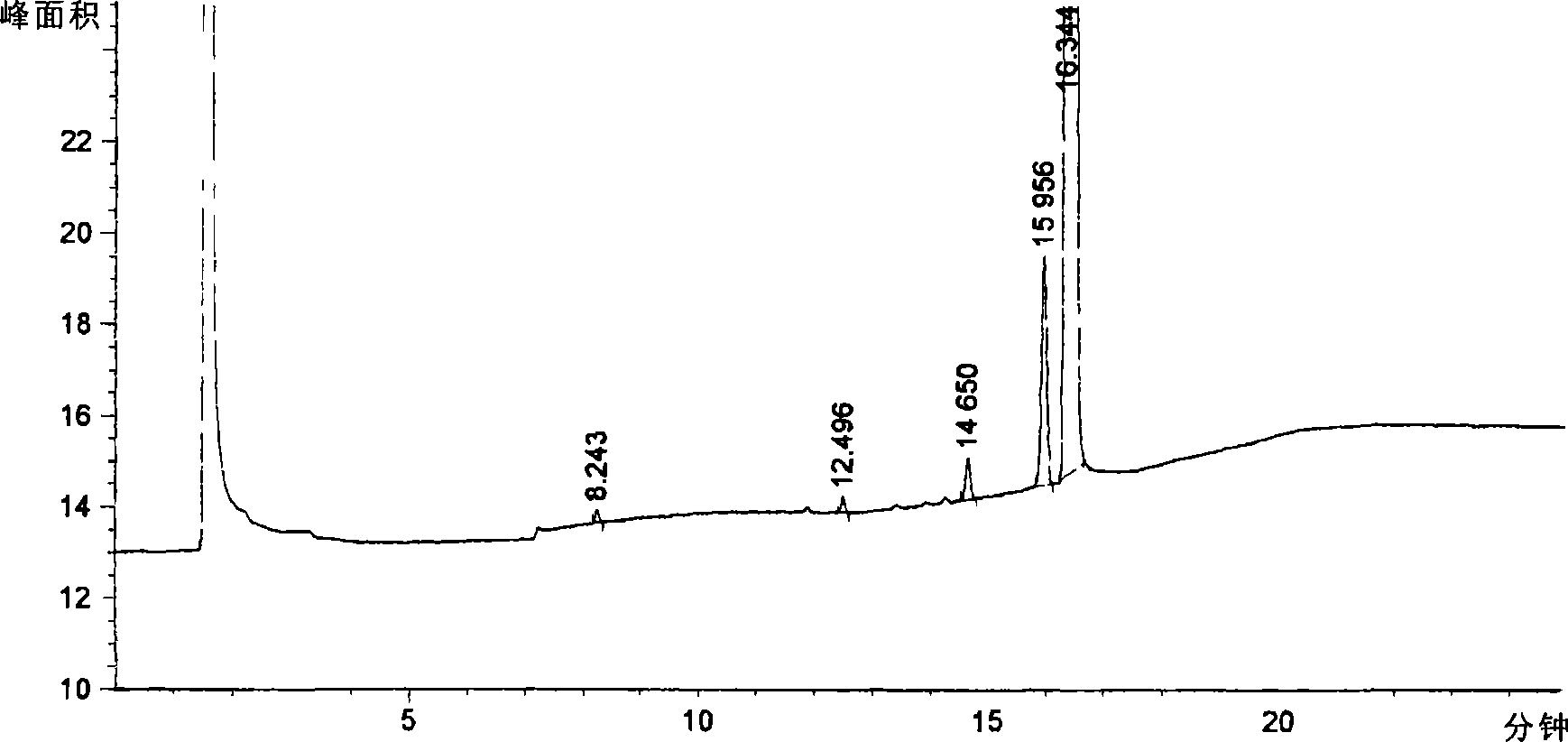
Method for preparing pinaverium bromide and application thereof
A kind of pinaverium bromide, reaction technology, applied in the field of preparation technology of pinaverium bromide, can solve the problem of low content of pinaverium bromide cis isomer etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
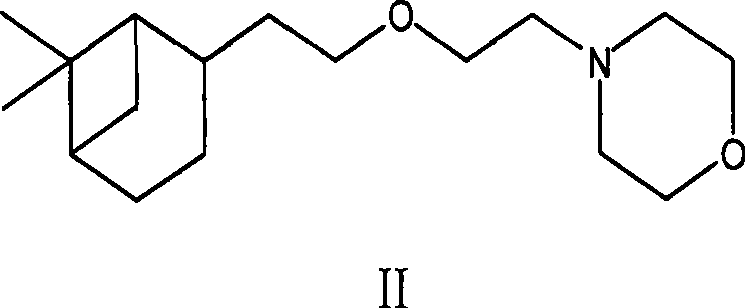
[0056] The preparation method of the intermediate compound shown in formula II provided by the present invention includes the steps:
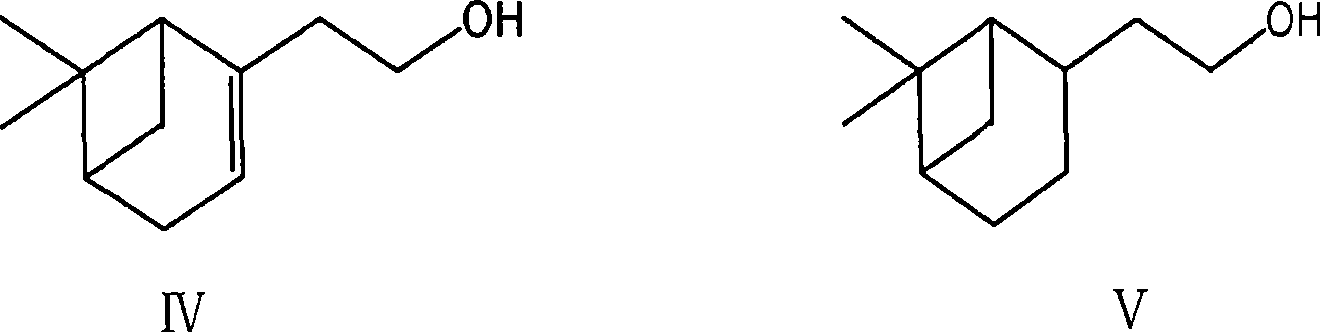
[0057] (a) Mixing reaction of nopol with a chemical structure of formula IV, palladium on carbon and methanol to obtain a compound of chemical structure with formula V;
[0058]
[0059] (b) Mixing the compound of formula V obtained in step (a), toluene and sodium hydroxide, and heating to react;
[0060] (c) Add a mixture of a compound with a chemical structure of formula VI and toluene, reflux, and cool to room temperature;
[0061] (d) Extract with water, take the organic layer, and distill under reduced pressure to obtain the compound intermediate of formula II.
[0062] A preferred solution is:
[0063] (a) Mixing nopol with a chemical structure of formula IV, palladium carbon and methanol to react for 2-8 hours, filtering, and removing methanol to obtain a compound with a chemical structure of formula V;
[0064] (b) Mixing the compound of fo...
Embodiment 1
[0098] Preparation of 2-[2-(2-morpholinyl-2-ethoxy-ethyl)]-6,6-dimethylnorphan (Formula II)
[0099] 1. At room temperature, put 600L of methanol, 100Kg of nopol (Formula IV), 10Kg of palladium on carbon in the autoclave, react for 4-6 hours under 6-7 kg of hydrogen pressure, filter the catalyst, wash with methanol, and evaporate under reduced pressure Methanol to obtain 90Kg of the compound of formula V with a yield of 88.7%;
[0100] 2. Then add 300L of toluene, 50Kg of compound of formula V into a 1000L reaction kettle, add 13kg of sodium hydroxide at room temperature, heat to reflux, and react for 2 hours;
[0101] 3. Then add 45kg of 2-chloroethylmorpholine (formula VI) and 100L of toluene mixture, reflux for 1 hour after addition, cool, add 300L of water for extraction, and separate the organic layer;
[0102] 4. The water layer was extracted twice with toluene, 200L each time, the organic layers were combined, washed with 200L water, and the toluene was evaporated under red...
Embodiment 2
[0106] Preparation of 2-[2-(2-morpholinyl-2-ethoxy-ethyl)]-6,6-dimethylnorphan (Formula II)
[0107] 1. At room temperature, put 600L of methanol, 100Kg of nopol (Formula IV), 10Kg of palladium on carbon in the autoclave, react for 2 hours under 9-10 kg of hydrogen pressure, filter the catalyst, wash with methanol, and evaporate the methanol under reduced pressure. 91.3Kg of compound of formula V was obtained, with a yield of 90.4%;
[0108] 2. Then add 300L of toluene, 50Kg of compound of formula V into a 1000L reaction kettle, add 13kg of sodium hydroxide at room temperature, heat to reflux, and react for 2 hours;
[0109] 3. Then add 45kg of 2-chloroethylmorpholine (formula VI) and 100L of toluene mixture, reflux for 1 hour after addition, cool, add 300L of water for extraction, and separate the organic layer;
[0110] 4. The water layer was extracted twice with toluene, 200L each time, the organic layers were combined, washed with 200L water, and the toluene was evaporated und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com