Method for preparing pinaverium bromide and application thereof
A kind of pinaverium bromide, reaction technology, applied in the field of preparation technology of pinaverium bromide, can solve the problem of low content of pinaverium bromide cis isomer etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The preparation method of the intermediate compound shown in formula II provided by the present invention comprises steps:
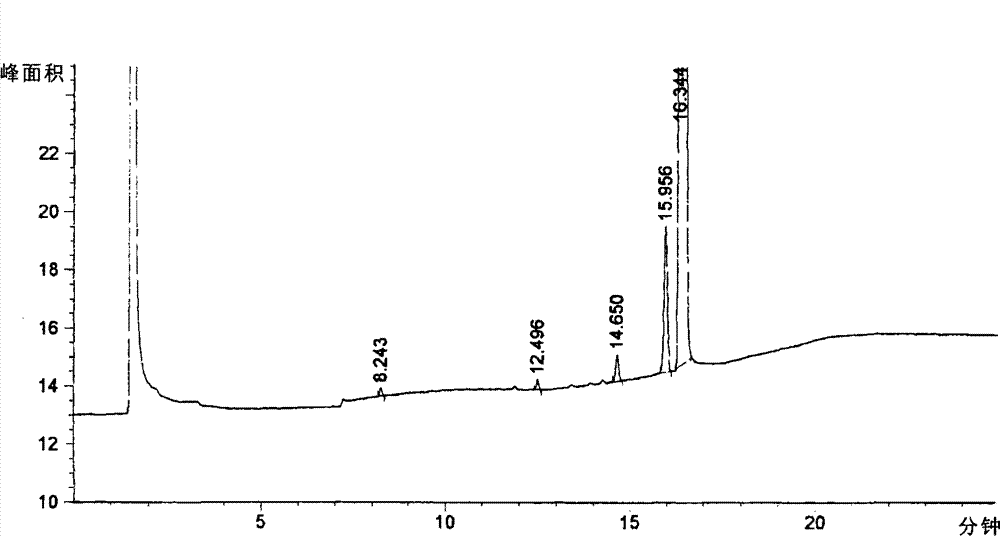
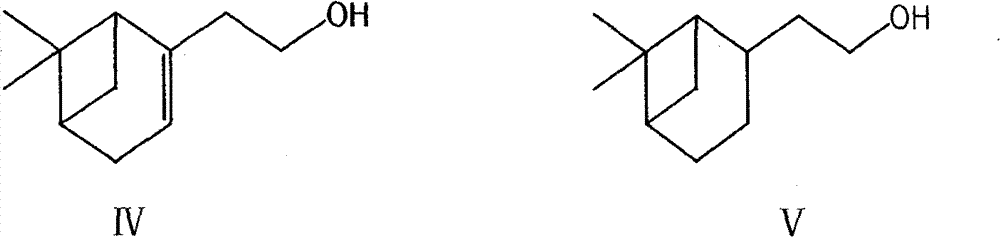
[0057] (a) Nopol alcohol, palladium carbon and methanol with chemical structure such as formula IV are mixed and reacted to obtain a compound with chemical structure such as formula V;
[0058]
[0059] (b) mixing the compound of formula V obtained in step (a), toluene and sodium hydroxide, and heating for reaction;
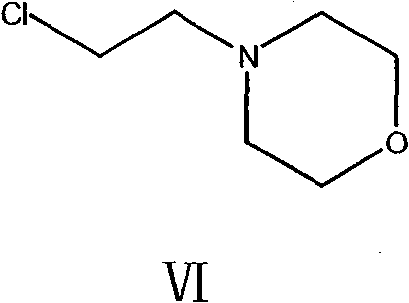
[0060] (c) Add the mixed solution of the compound of chemical structure such as formula VI and toluene, reflux, cool to room temperature;
[0061] (d) adding water for extraction, taking the organic layer, and distilling under reduced pressure to obtain the compound intermediate of formula II.
[0062] A preferred solution is:
[0063] (a) Nopol alcohol, palladium carbon and methanol with chemical structure such as formula IV were mixed and reacted for 2-8 hours, filtered, and methanol was removed to obtain a compound with chemica...
Embodiment 1
[0098] Preparation of 2-[2-(2-morpholinyl-2-ethoxyl-ethyl)]-6,6-dimethylnoprene (formula II)
[0099] 1. At room temperature, put into the autoclave 600L of methanol, 100Kg of Nopol alcohol (formula IV), 10Kg of palladium carbon, react under 6-7 kg of hydrogen pressure for 4-6 hours, filter the catalyst, wash with methanol, and evaporate under reduced pressure Methanol to obtain 90Kg of the compound of formula V, with a yield of 88.7%;
[0100] 2. Then add 300L of toluene and 50Kg of the compound of formula V into the 1000L reactor, put in 13kg of sodium hydroxide at room temperature, raise the temperature to reflux, and react for 2 hours;
[0101] 3. Then add the mixed solution of 45kg 2-chloroethylmorpholine (formula VI) and 100L toluene, reflux reaction for 1 hour after adding, cool, add 300L water for extraction, and separate the organic layer;
[0102] 4. The aqueous layer was extracted twice with toluene, 200 L each time, the organic layers were combined, washed with 20...
Embodiment 2
[0106] Preparation of 2-[2-(2-morpholinyl-2-ethoxyl-ethyl)]-6,6-dimethylnoprene (formula II)
[0107] 1. Under room temperature, drop into methanol 600L in the autoclave, Nopol alcohol 100Kg (formula IV), palladium carbon 10Kg, react 2 hours under 9-10 kilograms of hydrogen pressure, filter catalyst, wash with methanol, decompress and evaporate methyl alcohol, 91.3Kg of the compound of formula V was obtained, with a yield of 90.4%;
[0108] 2. Then add 300L of toluene and 50Kg of the compound of formula V into the 1000L reactor, put in 13kg of sodium hydroxide at room temperature, raise the temperature to reflux, and react for 2 hours;
[0109] 3. Then add the mixed solution of 45kg 2-chloroethylmorpholine (formula VI) and 100L toluene, reflux reaction for 1 hour after adding, cool, add 300L water for extraction, and separate the organic layer;
[0110] 4. The aqueous layer was extracted twice with toluene, 200 L each time, the organic layers were combined, washed with 200 L ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com