Double platinum (II) pyridine complex of organic photoelectric materials, synthetic method and applications
A synthesis method and technology of complexes, which are applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of low luminescence quantum yield restriction and short excited state lifetime.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
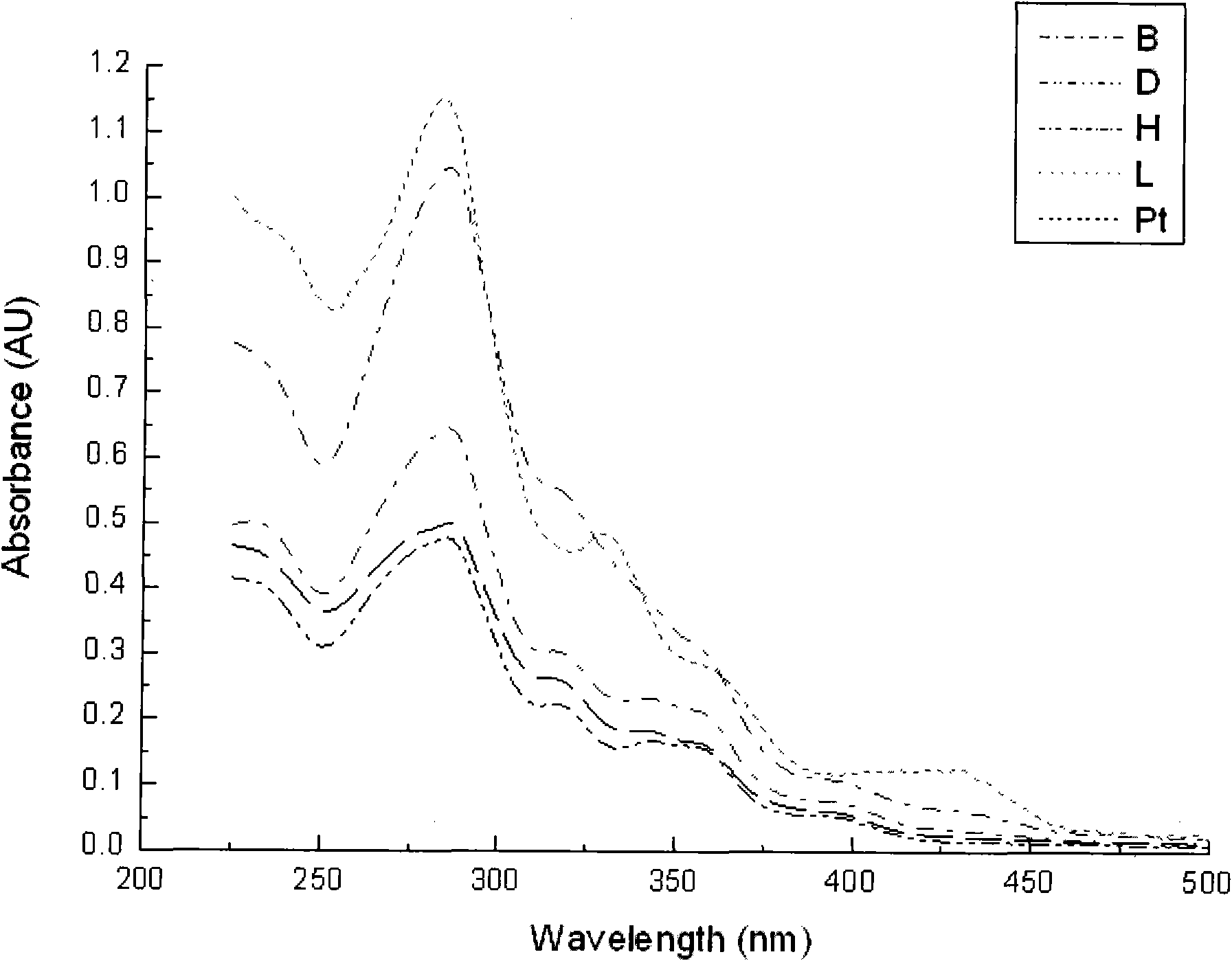
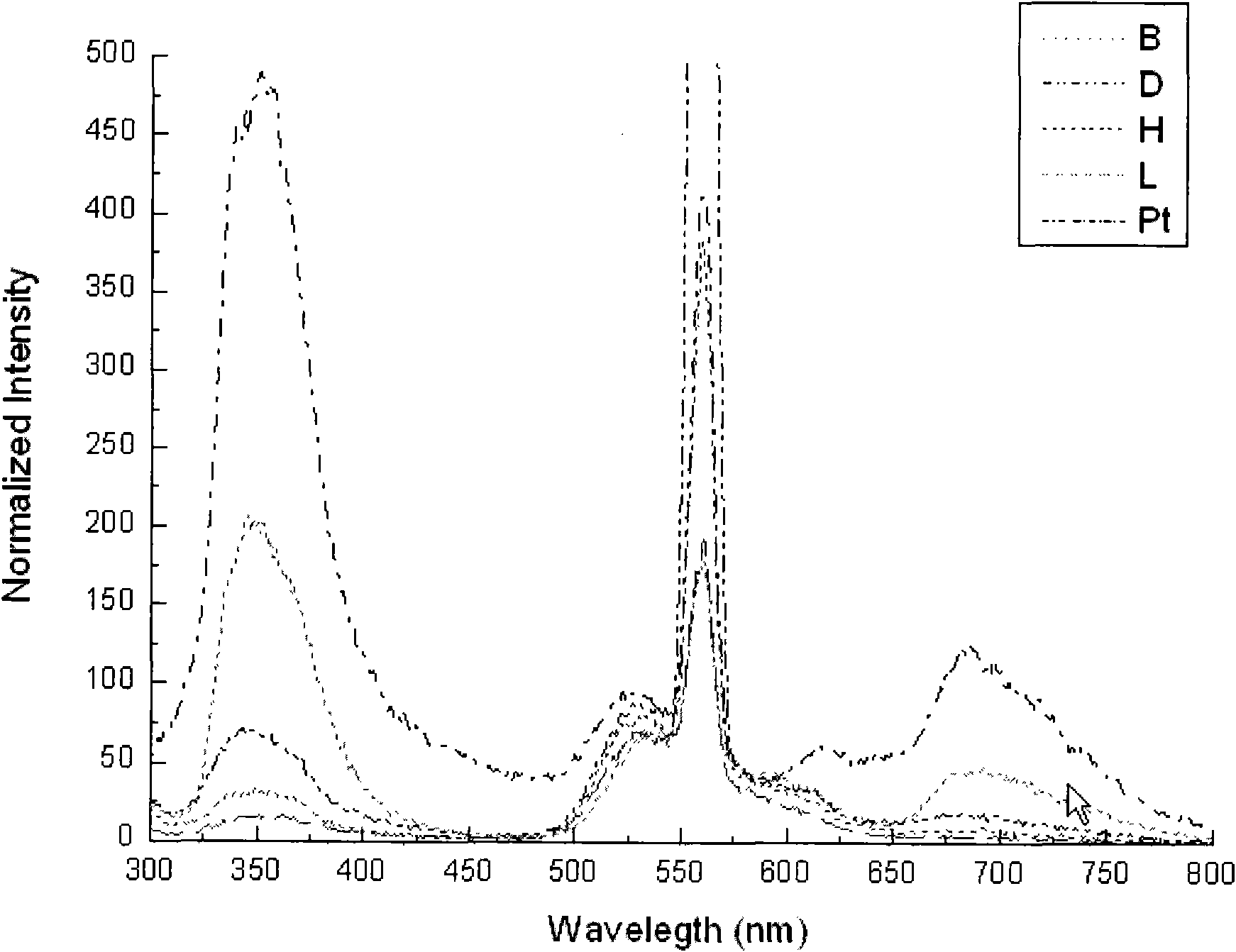
[0015] Add 0.1482g (0.5mmol) of 4,6-diphenyl-2,2'-bipyridine, 0.2003g (0.5mmol) of potassium platinite chloride, 20mL of acetonitrile, and 20mL of distilled water into a 100mL round bottom flask. Magnetic stirring, heating in an oil bath, reflux for 48 hours, distilled off the solvent under reduced pressure, added 5 mL of dichloromethane to dissolve the product, filtered, added diethyl ether to the filtrate, and precipitated orange solid 4,6-diphenyl-2,2'-di Pyridinium chloride (II) (Pt), the yield is 70.6%. 1 H NMR (DMSO-d 6 , 300MHz) δ: 7.11-7.18(m, 2H), 7.51-7.54(d, 1H), 7.60-7.63(m, 3H), 7.84-7.86(d, 1H), 7.93-7.97(t, 1H), 8.12-8.15(t, 2H), 8.31(s, 1H), 8.37-8.42(m, 1H), 8.54-8.55(d, 1H), 8.77-8.79(d, 1H), 8.94-8.95(d, 1H) ).ESI-MS: The calculated value is [C 22 h 15 N 2 Pt] + , 502.4; the measured value is 601.9. Elemental analysis: the calculated value is C, 49.12; H, 2.81; N, 5.21. The measured value is C, 49.05; H, 2.78; N, 5.01. UV absorption spectrum see attac...
Embodiment 2
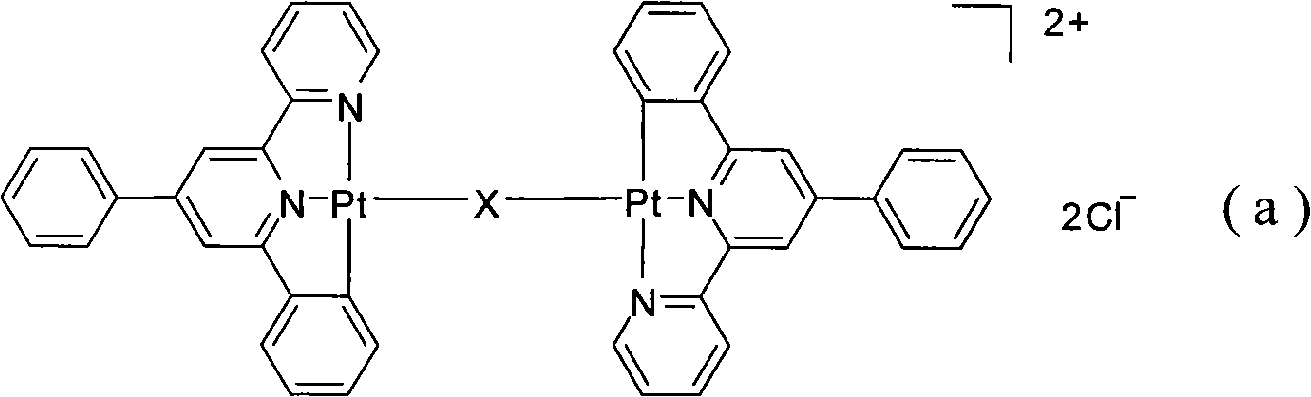
[0017] 0.5379g (1.0mmol) of 4,6-diphenyl-2,2'-bipyridylplatinum chloride (II), 0.1282g (0.5mmol) of N,N'-bisisonicotinylmethylenediamine , 500mL of acetonitrile and 500mL of distilled water were added to a 2000mL round bottom flask. Magnetic stirring, reacted at 25°C for 48 hours, distilled off the solvent under reduced pressure, added 25mL of dichloromethane to dissolve the product, filtered, added diethyl ether to the filtrate, and precipitated orange solid bisplatinum (II) pyridine complex dichloride μ-(N,N '-Bisisonicotinylmethylenediamine)bis[(4,6-diphenyl-2,2'-bipyridyl)platinum(II)]A 0.45g, the yield was 68.2%.
Embodiment 3
[0019] 0.5379 g (1.0 mmol) of 4,6-diphenyl-2,2'-bipyridine chloride platinum (II) complex, 0.1351 g of N,N'-bisisonicotinyl-1,2-ethylenediamine g (0.5 mmol), 500 mL of acetonitrile, and 500 mL of distilled water were added to a 2000 mL round bottom flask. Magnetic stirring, reacted at 25°C for 48 hours, distilled off the solvent under reduced pressure, added 25mL of dichloromethane to dissolve the product, filtered, added diethyl ether to the filtrate, and precipitated orange solid bisplatinum (II) pyridine complex dichloride μ-(N,N '-Bisonicotinoyl-1,2-ethylenediamine)bis[(4,6-diphenyl-2,2'-bipyridyl)platinum(II)]B 0.41g, yield 61.5%. 1 H NMR (DMSO-d 6 , 300MHz) δ: 3.47(s, 4H), 7.06-7.17(m, 4H), 7.50(d, 2H), 7.59-7.61(m, 6H), 7.74(d, 4H), 7.81(d, 2H) , 7.91(t, 2H), 8.10-8.13(m, 4H), 8.25(s, 2H), 8.36(t, 2H), 8.50(s, 2H), 8.71-8.75(m, 6H), 8.90(d , 4H).ESI-MS: The calculated value is [C 62 h 52 N 8 o 2 Pt 2 ] 2+ , 637.6; found value is 637.7. Elemental analysis: calcu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com