Reagent kit for detecting agedness yellow spot degenerative disease
A technology for degenerative diseases and age-related macular, which can be used in measurement devices, microbial determination/inspection, instruments, etc., and can solve problems such as poor results and difficult vision functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] [Example 1] Blood sample collection and specimen processing
[0041] A total of 214 Chinese patients with age-related macular degeneration (AMD) and 214 controls were selected. The patients were clinically confirmed AMD, including 115 patients with wet AMD and 99 patients with dry AMD. The controls were people who were older than or equal to 60 years old, had no drusen and retinal pigment changes, and matched the age group of AMD patients.
[0042] For all participants, a detailed investigation of their medical and family histories, and a physical examination and detailed ophthalmology specialist examination were performed. After signing the informed consent form, 5-10 ml of EDTA anticoagulated blood samples were collected from each person. Genomic DNA was extracted by the phenol-chloroform method.
Embodiment 2
[0043] [Example 2] PCR amplification and genotype analysis of the deletion / insertion variant gene at 4340 bases upstream of the HTRA1 gene
[0044] (1) Electrophoretic separation and identification of PCR amplification products
[0045] 1. Take the blood of the subject and extract the DNA;
[0046] 2. With 5'-GCCAACTGGAGCTTCTCATC-3' and
[0047] 5'-GTAGTTTCCAGGGGCTCTCC-3' is the upstream and downstream primers for PCR amplification of genomic DNA; reaction conditions:
[0048] 95℃3minutes, (94℃30seconds, 57℃30seconds, 72℃45seconds)*35cycles
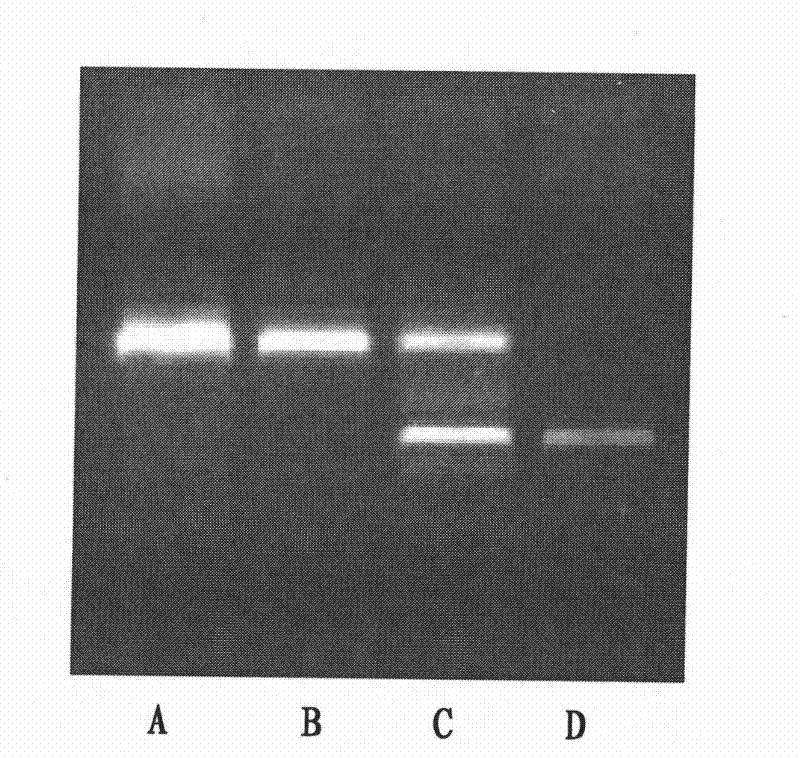
[0049] 3. Separation by 1% agarose gel electrophoresis, and discrimination of deletion / insertion variation based on the size of the PCR product.
[0050] Agarose electrophoresis identification:
[0051] The PCR product containing the deletion / insertion sequence of 4340bp upstream of the HTRA1 gene was separated by 1% agarose gel electrophoresis. The deletion / insertion made the normal fragment of the PCR product about 400bp smaller, an...
Embodiment 3
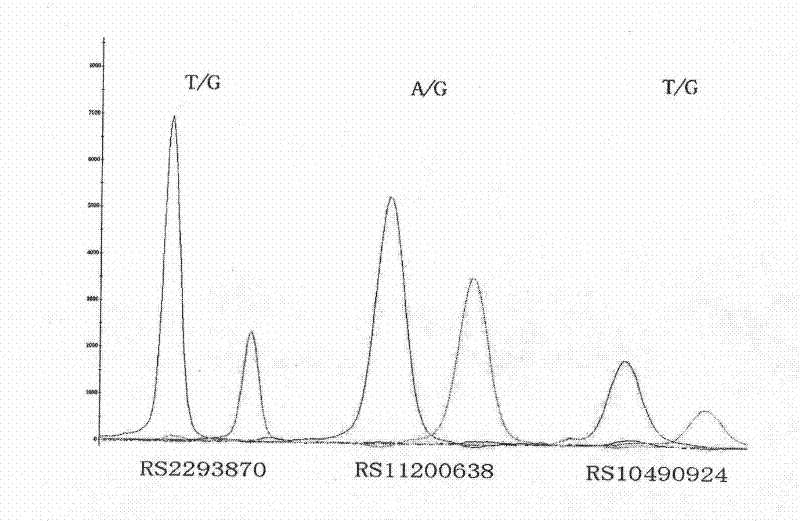
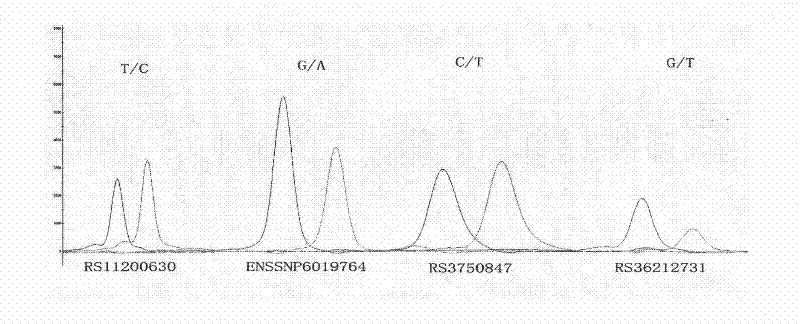
[0086] [Example 3] PCR amplification and genotype analysis of DNA fragments containing rs2293870, rs11200638, and rs10490924 single nucleotide polymorphisms
[0087] 1. Extract the blood genome DNA of AMD patients and control groups.
[0088] 2. PCR amplification of genomic DNA fragments containing target sequences (rs2293870, rs11200638, rs10490924)
[0089] Primer sequence:
[0090] rs2293870: Forward: 5'-GGCCGCTCGGCGCCTTTGGC-3'
[0091] Reverse: 5'-CCCCGAAGGGCACCACGCAC-3'
[0092] rs11200638: Forward: 5'-ATGCCACCCACAACAACTTT-3'
[0093] Reverse: 5'-CGCGTCCTTCAAACTAATGG-3'
[0094] rs10490924: Forward: 5'-TACCCAGGACCGATGGTAAC-3';
[0095] Reverse: 5'-GAGGAAGGCTGAATTGCCTA-3'
[0096] Reaction conditions:
[0097] rs2293870: 95℃3minutes, (94℃30seconds, 57℃30seconds, 72℃45seconds)*35cycles
[0098] rs11200638: 95℃3minutes, (94℃30seconds, 52℃30seconds, 72℃45seconds)*35cycles
[0099] rs10490924: 95℃3minutes, (94℃30seconds, 55℃30seconds, 72℃45seconds)*35cycles
[0100] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com