Method for purifying RNA on preparative scale by means of HPLC
An alkylation and mobile phase technology, applied in DNA preparation, recombinant DNA technology, organic chemistry, etc., can solve complex and expensive gradient procedures and other problems, and achieve the effects of simple recovery, sensitive detection, and easy automatic operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
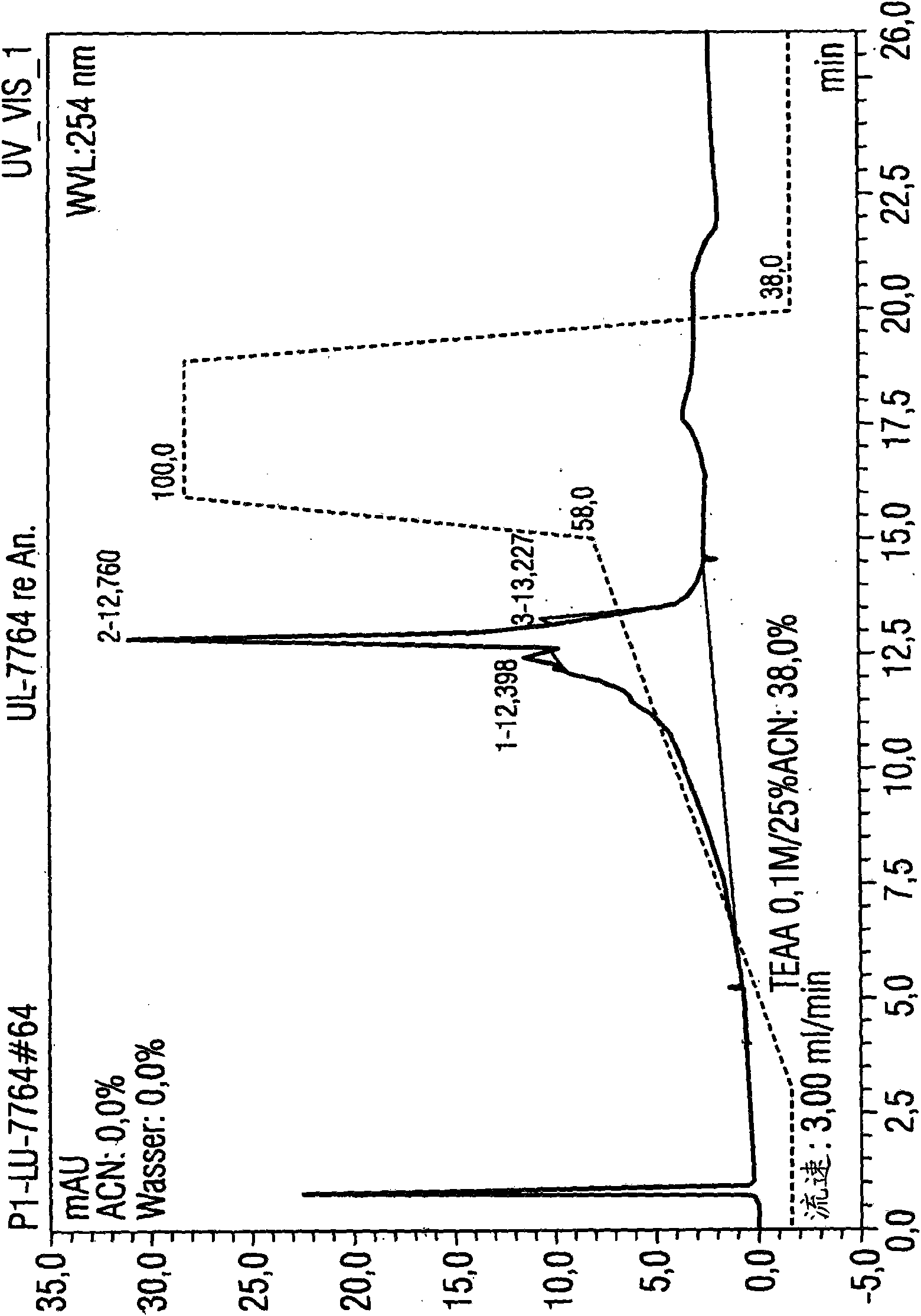
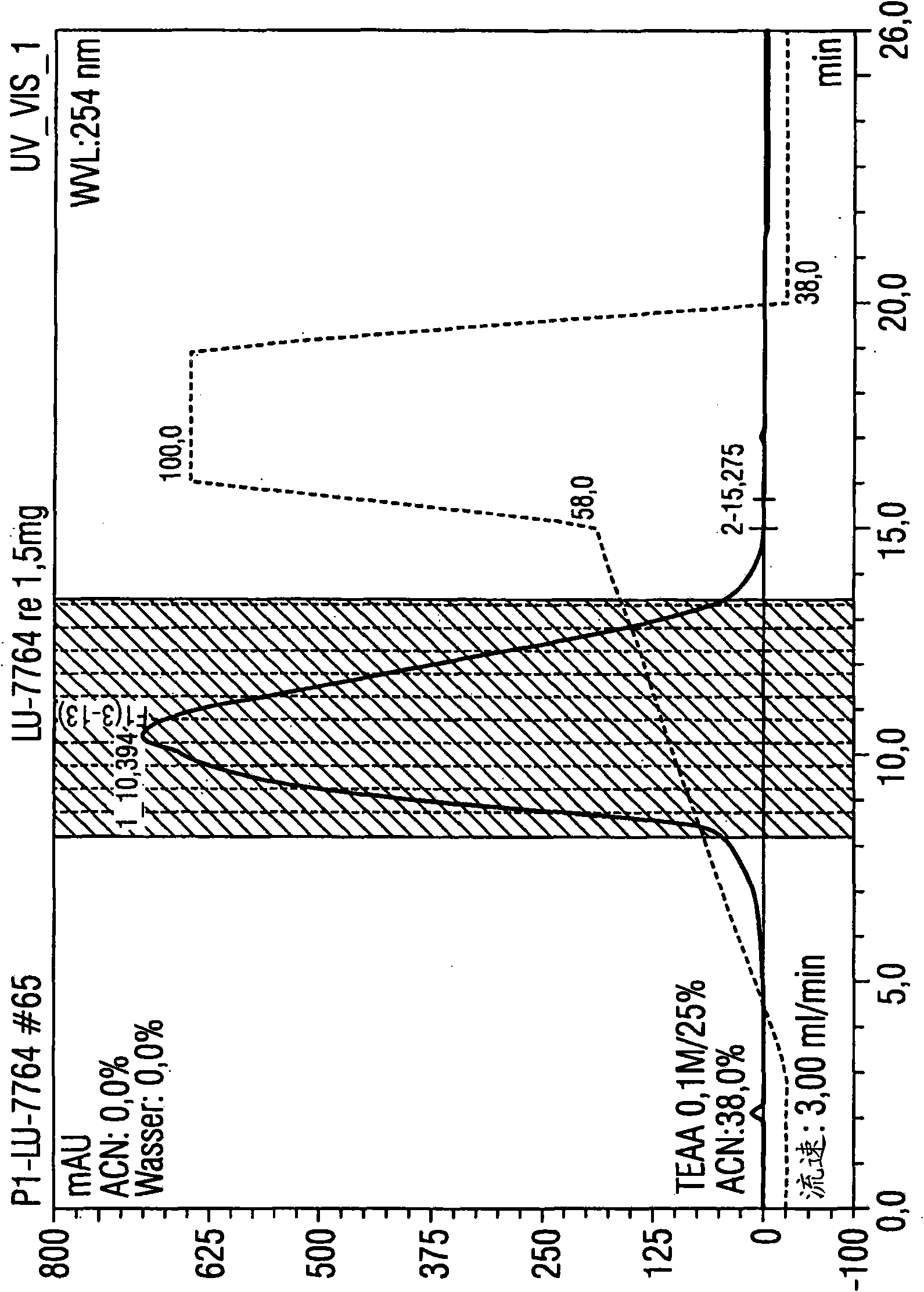
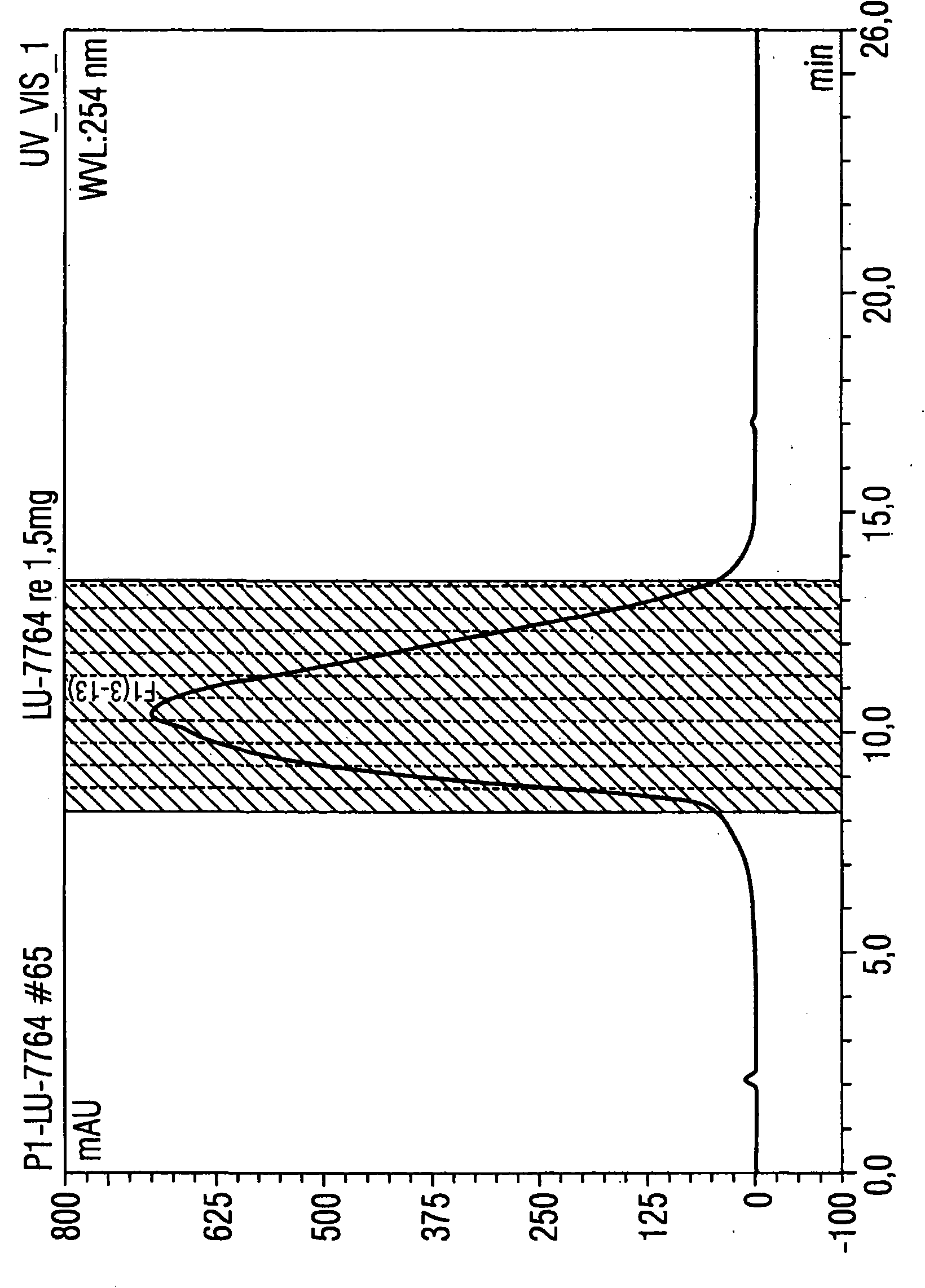
[0088] Example 1: Utilize the HPLC method according to the present invention to purify 1.5 mg luciferase mRNA
[0089] Luciferase mRNA with a size of 1825 base pairs was used for isolation. A porous non-alkylated polystyrene / divinylbenzene (polystyrene divinylbenzene) matrix (commercially available from Polymer Laboratories) was used as the stationary phase. Its particle size is 8 μm and its pore size is The columns used were 5.0 cm in length and 7.5 mm in diameter. The temperature of the injector was 12°C, and the temperature of the HPLC separation, specifically, the temperature of the separation column was 78°C, that is, the operation was performed under completely denaturing conditions.
[0090] Separation was performed by the following gradient program:
[0091] Eluent A: 0.1M triethylammonium acetate, pH7
[0092] Eluent B: 0.1M triethylammonium acetate, pH7, containing 25vol.% acetonitrile
[0093] Eluent composition:
[0094] Start: 62% A and 38% B (1st to 3rd ...
Embodiment 2
[0104] Example 2: Use aperture as The stationary phase separates 200 μg of 2kb and 4kb RNA fragments
[0105] 200 μg of 2kb and 4kb RNA fragments were used for isolation. A porous non-alkylated polystyrene / divinylphenyl substrate (commonly available from Polymer Laboratories) was used as stationary phase. Its particle size is 8 μm and its pore size is The columns used were 2.5 cm long and 4.6 mm in diameter. The injector temperature was 12°C, and the HPLC separation temperature, specifically, the temperature of the separation column was 78°C, that is, the operation was performed under completely denaturing conditions.
[0106] Separation was performed using the following gradient program:
[0107] Eluent A: 0.1M triethylammonium acetate
[0108] Eluent B: 0.1M triethylammonium acetate / 25% acetonitrile
[0109] Eluent composition:
[0110] · Starting level: 62% A and 38% B (1st to 3rd minutes)
[0111] Separation range I: Gradient 38%-49.5% B (B increase 5.75% / min) ...
Embodiment 3
[0116] Example 3: Use aperture as The stationary phase separates 100 μg of 2kb and 4kb RNA fragments
[0117] 100 μg of 2kb and 4kb RNA fragments were used for isolation. A porous non-alkylated polystyrene / divinylphenyl substrate (commonly available from Polymer Laboratories) was used as stationary phase. Its particle size is 8 μm and its pore size is The columns used were 2.5 cm in length and 4.6 mm in diameter. The injector temperature was 12°C, and the HPLC separation temperature, specifically, the temperature of the separation column was 78°C, that is, the operation was performed under completely denaturing conditions.
[0118] Separation was performed using the following gradient program
[0119] Eluent A: 0.1M triethylammonium acetate
[0120] Eluent B: 0.1M triethylammonium acetate / 25% acetonitrile
[0121] Eluent composition:
[0122] · Starting level: 62% A and 38% B (1st to 3rd minute)
[0123] Separation range I: Gradient 38%-49.5% B (B increase 5.75% / min...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com