Intraocular drug delivery systems
A technology of delivery system and drug, applied in the field of drug delivery system for the treatment of eye diseases, can solve the problems of increasing healing time, patient discomfort and the risk of infection or other complications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] 2-Methoxyestradiol Polylactic Acid Microspheres (Rabbit)
[0150] In this experiment, PLA-based microspheres containing the active agent 2-methoxyestradiol were prepared and administered by injection into various intraocular sites in rabbit eyes. Remarkably, we found that the microspheres were well tolerated and all eye redness (eye surface hyperemia) disappeared (disintegration) within one week after intraocular administration. Thus, administration of these microspheres by the methods described herein does not result in significant hyperemia of the inner surface of the eye.
[0151] The microspheres used in this experiment contained 29.7 wt% of 2-methoxyestradiol as the therapeutic agent and 70.3 wt% of poly(D,L) lactic acid polymer (Birmingham) with an intrinsic viscosity (iv) of 1.2 dL / gm. Before administration, the microspheres were suspended in isotonic phosphate buffered saline (IPBS) at pH 7.4 as the microsphere carrier. This microsphere suspension containe...
Embodiment 2
[0172] Subconjunctival brimonidine polylactic acid microspheres (rabbit)
[0173] Two experiments were performed in which lactic acid polymer, brimonidine therapeutic agent microspheres were prepared and injected into the subconjunctival space of rabbit eyes with a syringe. PLA brimonidine microspheres are injected as a suspension in an aqueous carrier such as isotonic phosphate buffered saline. The viscosity of the microsphere suspension is up to about 200,000 cps at 20°C. Brimonidine-containing microspheres are injected subconjunctivally (with a 25-30 gauge syringe needle) to deliver therapeutic levels of the therapeutic agent (brimonidine) in the anterior chamber (for example, to treat ocular hypertension) and / or the posterior chamber of the eye A therapeutic level of the therapeutic agent (brimonidine) is provided for the treatment of retinal diseases.
[0174] Notably, PLA microspheres containing brimonidine were well tolerated in both experiments (no hyperemia after ...
Embodiment 3
[0192] Intraocular Brimonidine PLA Implant (Rabbit)
[0193] I. Subfascial Implantation
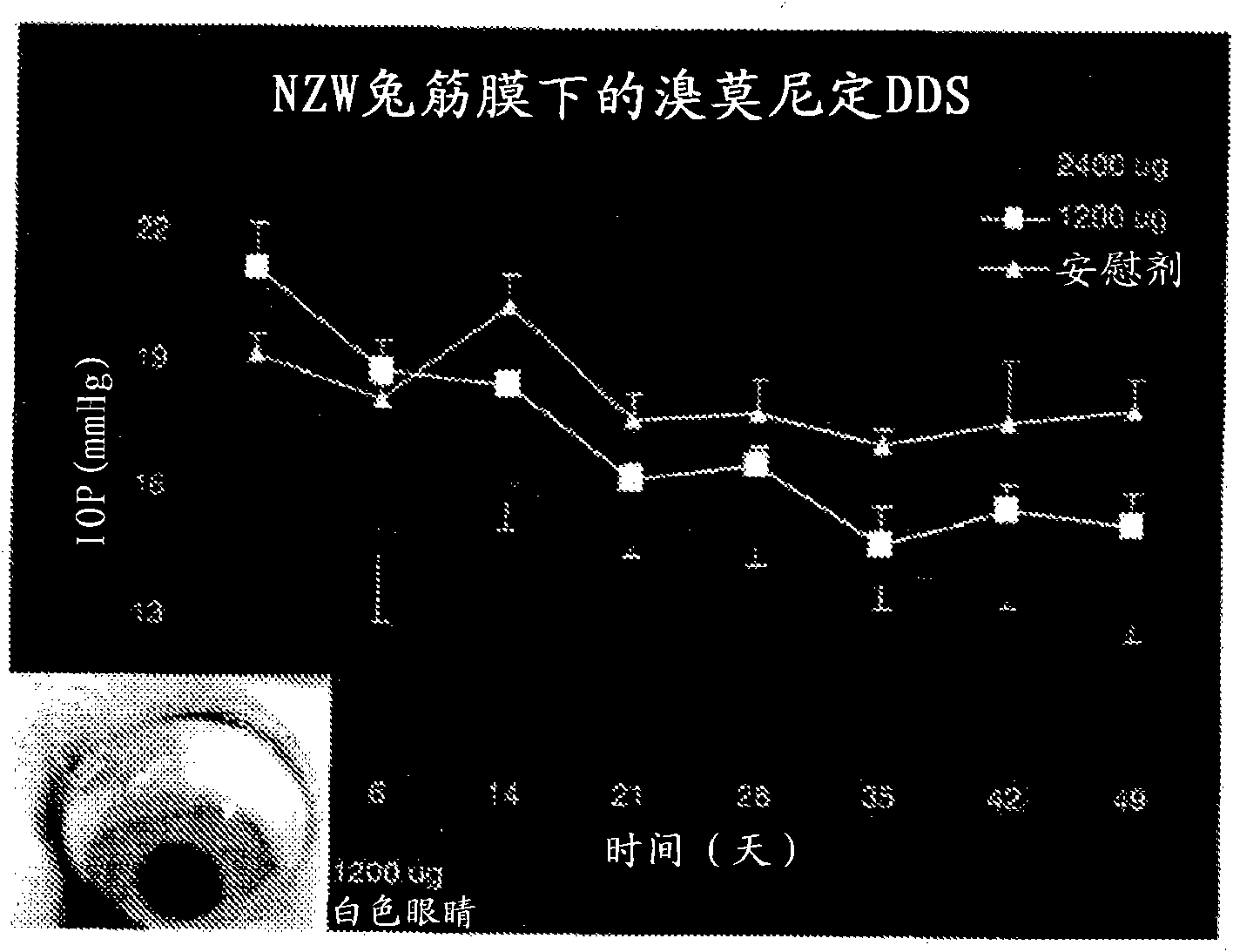
[0194] A. In the first experiment, brimonidine tartrate PLA implants were implanted in the anterior subfascial space. 3 or 6 brimonidine tartrate implants (each implant containing 400 μg brimonidine tartrate) were implanted in the anterior subfascial region ( implant placement as figure 1 shown in the lower right photo). Three implants (total dose 1200 μg) were placed in 3 rabbits, 6 implants (total dose 2400 μg) were placed in 3 rabbits, and placebo implants were also placed in 3 rabbits.
[0195] figure 1 Placement of the implant was shown to result in a reduction in intraocular pressure within 50 days, with a dose response occurring. IOP was measured with a barometric tonometer.
[0196] The implant was placed in the anterior subfascial space by grasping the conjunctiva approximately 3-4 mm posterior to the limbus with toothed forceps. Wescott scissors were used to enter the c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com