Method for converting diester-type and ester-type aconite alkaloids into monoester pyrolysis-type alkaloids
A technology for alkaloids and aconitum, which is applied in the field of monoester pyrolysis alkaloids, and can solve problems such as poor reproducibility, poor water solubility, and low conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
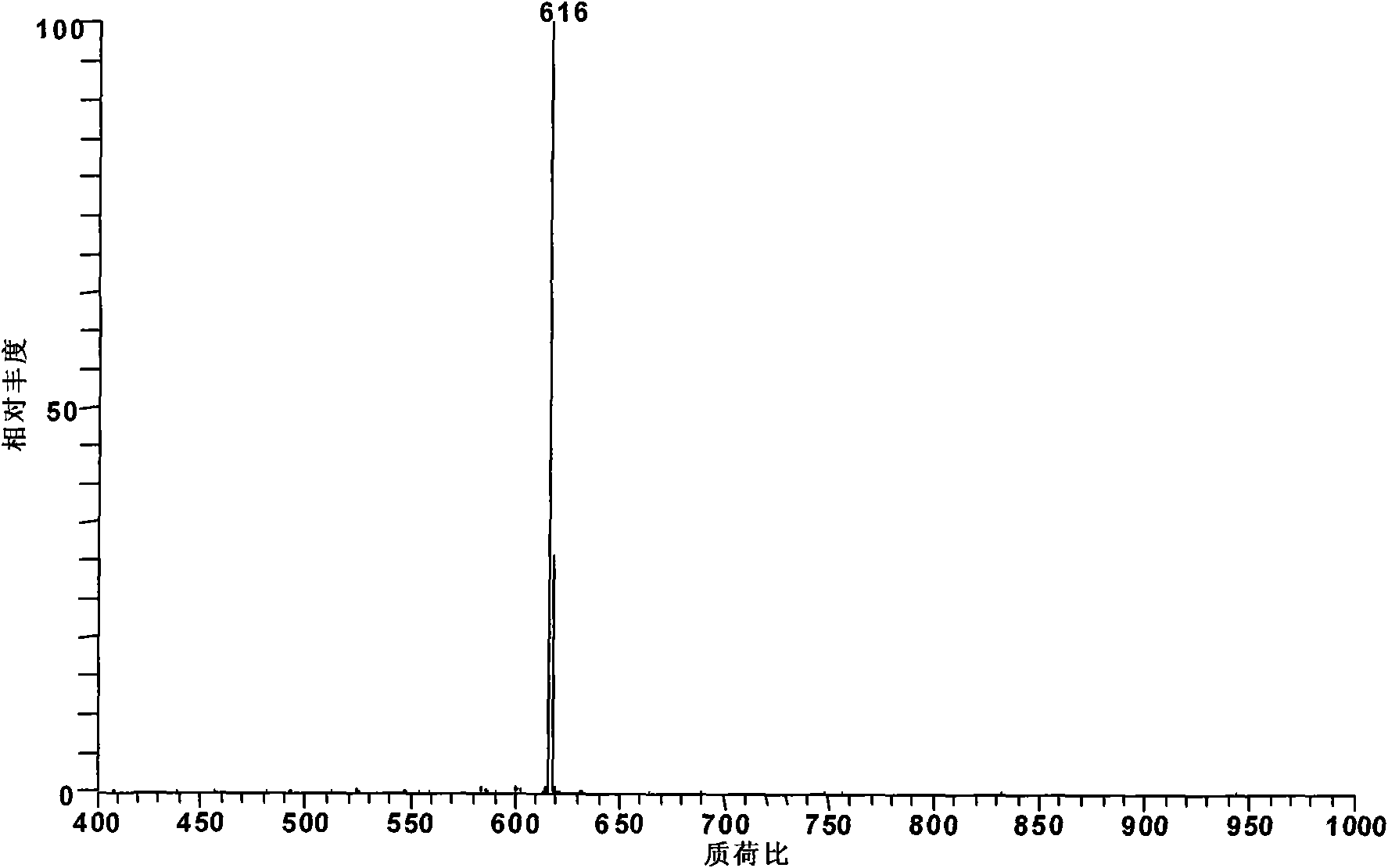
[0017] Add 1 mg of mesoaconitine standard substance into 100 ml of pyridine. At this time, pyridine is submerged in the added standard substance, and heated to 115° C. and boiled and refluxed for 2 hours to obtain monoester pyrolysis type alkaloid deacetic acid mesoaconitine (m / z 572).
[0018] The obtained reaction product was diluted 100 times with methanol and then detected by electrospray mass spectrometry. According to its relative abundance ratio equal to the molar concentration ratio in electrospray mass spectrometry, the conversion rate of aconitine was 91%. The alkaloid components in the system after the reaction were deacetated mesoaconitine (m / z 572) and incompletely reacted mesoaconitine (m / z 632).
Embodiment 2
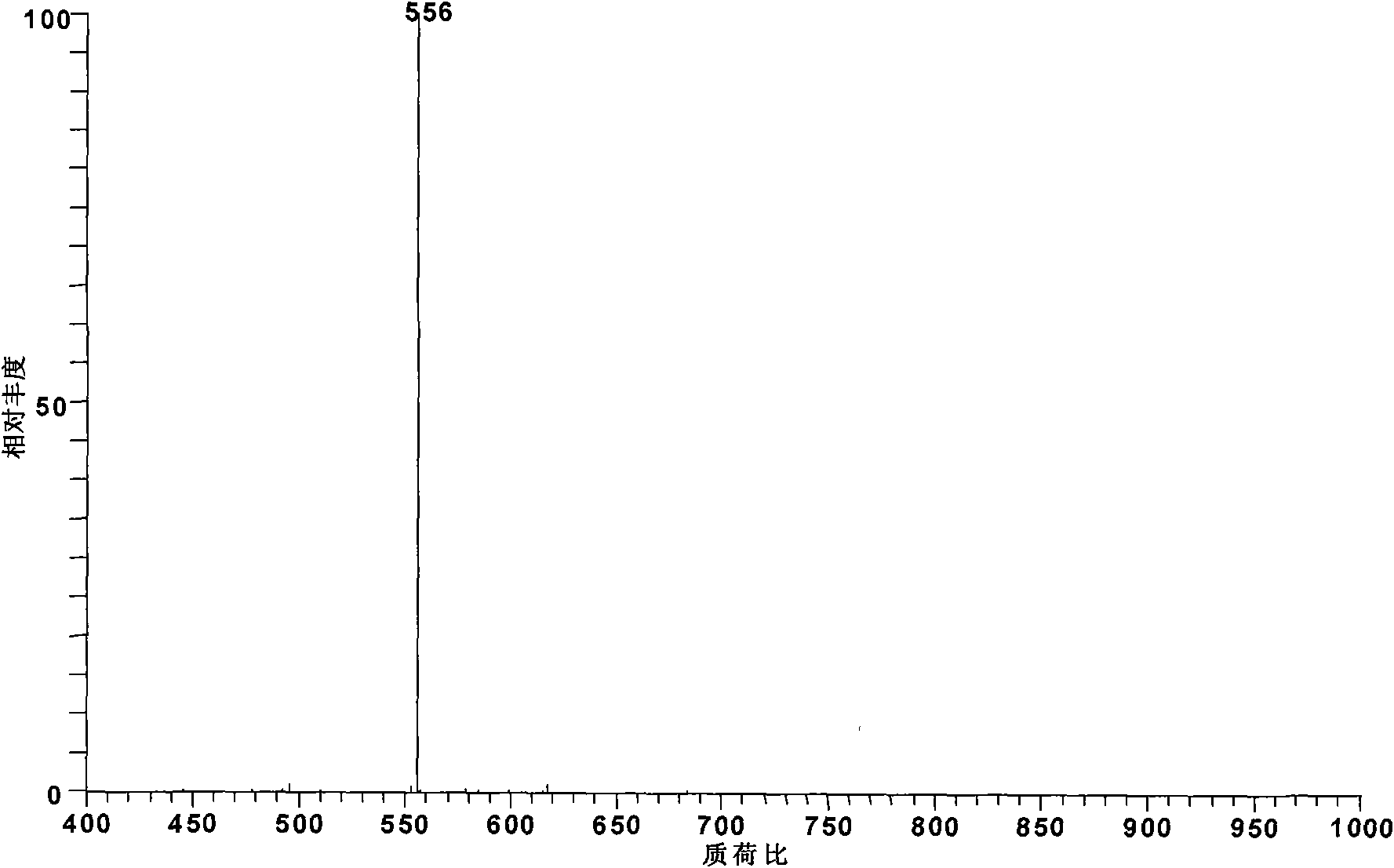
[0020] Add 1 mg of mesoaconitine standard substance into 100 ml of pyridine. At this time, the pyridine is submerged in the added standard substance, and heated to 115° C. and boiled and refluxed for 6 hours to obtain monoester pyrolysis type alkaloid deacetic acid mesoaconitine (m / z 572).
[0021] The obtained reaction product was diluted 100 times with methanol and then detected by electrospray mass spectrometry. According to its relative abundance ratio equal to the molar concentration ratio in electrospray mass spectrometry, the conversion rate of aconitine was 92%. The alkaloid components in the system after the reaction were deacetated mesoaconitine (m / z 572) and incompletely reacted mesoaconitine (m / z 632).
Embodiment 3
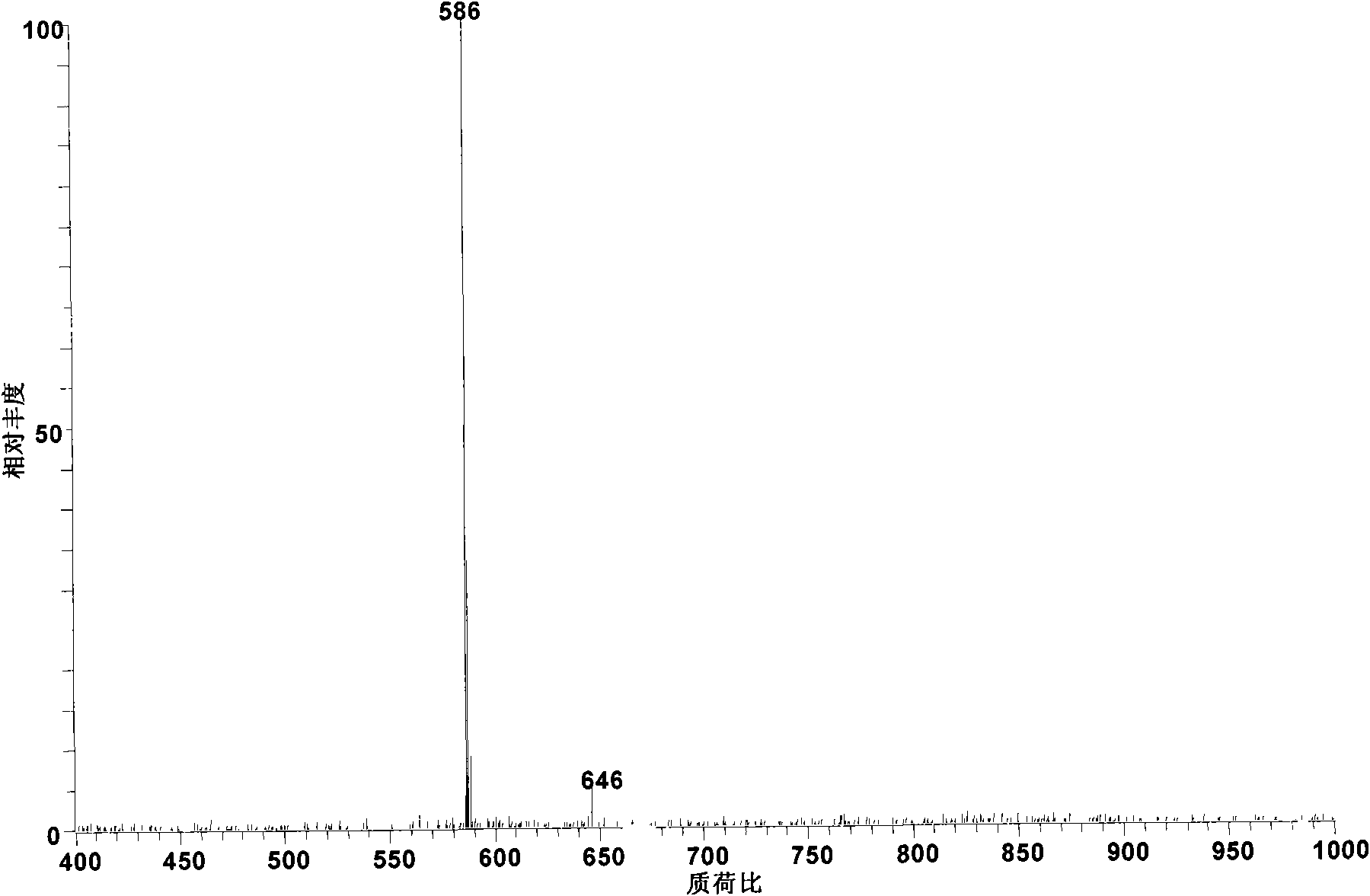
[0023] Add 1 mg of the standard substance of aconitine to 100 ml of pyridine. At this time, the pyridine was submerged in the standard substance added, and heated to 115° C. and boiled and refluxed for 8 hours to obtain the monoester pyrolysis type alkaloid deacetic acid, aconitine (m / z 572).
[0024] The obtained reaction product was diluted 100 times with methanol and then detected by electrospray mass spectrometry. According to its relative abundance ratio equal to the molar concentration ratio in electrospray mass spectrometry, the conversion rate of aconitine was 93%. The alkaloid components in the system after the reaction were deacetated mesoaconitine (m / z 572) and incompletely reacted mesoaconitine (m / z 632).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com