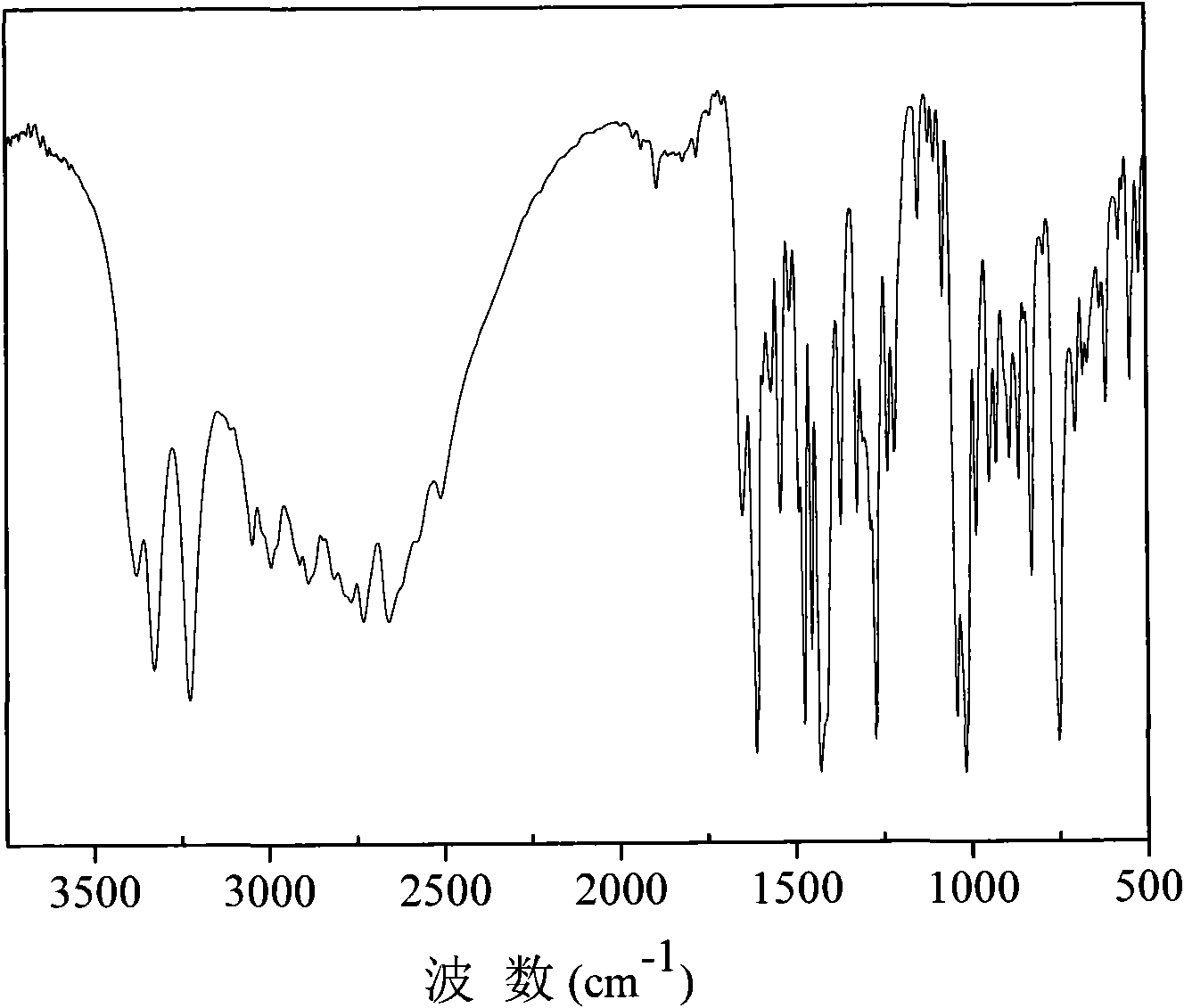
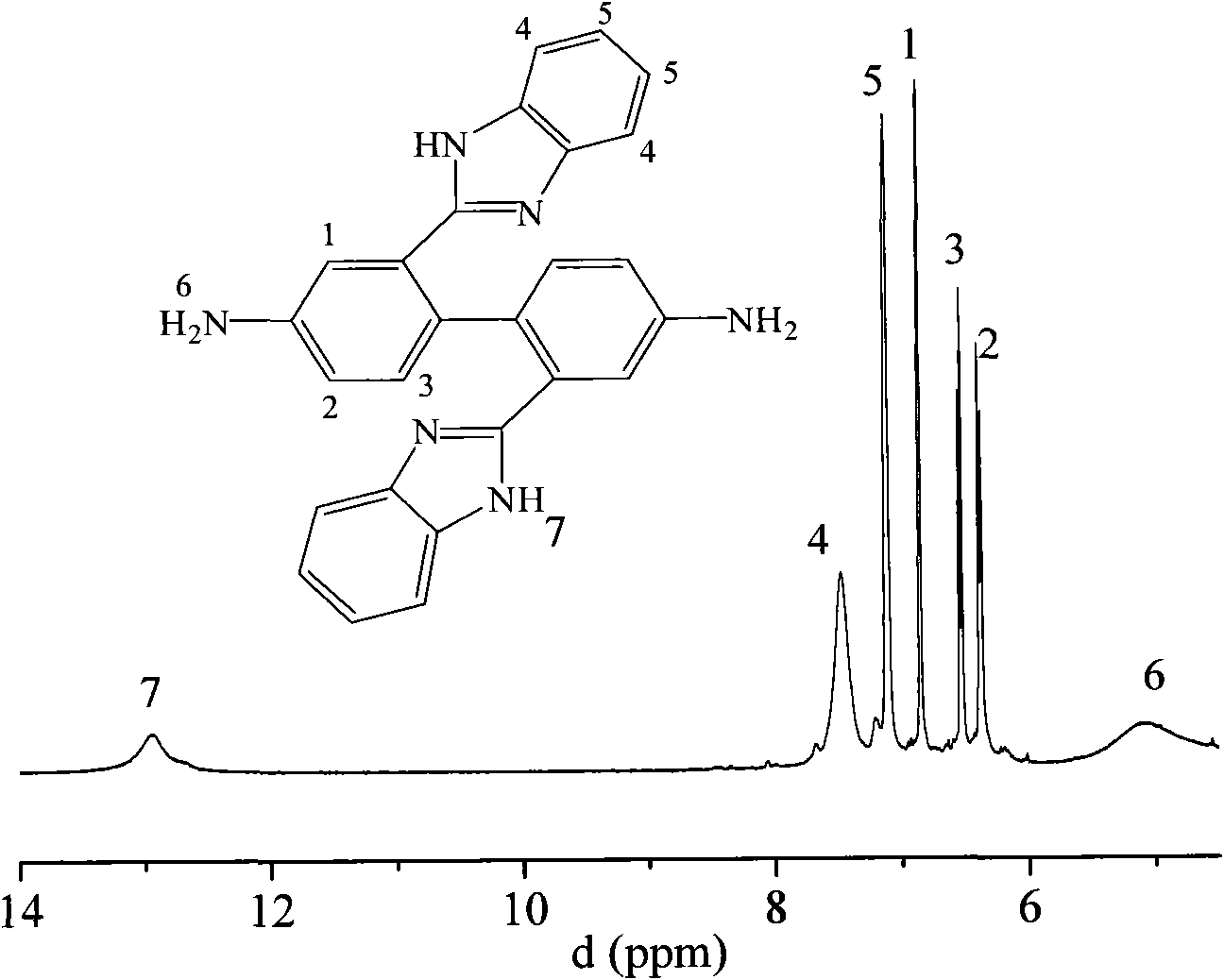
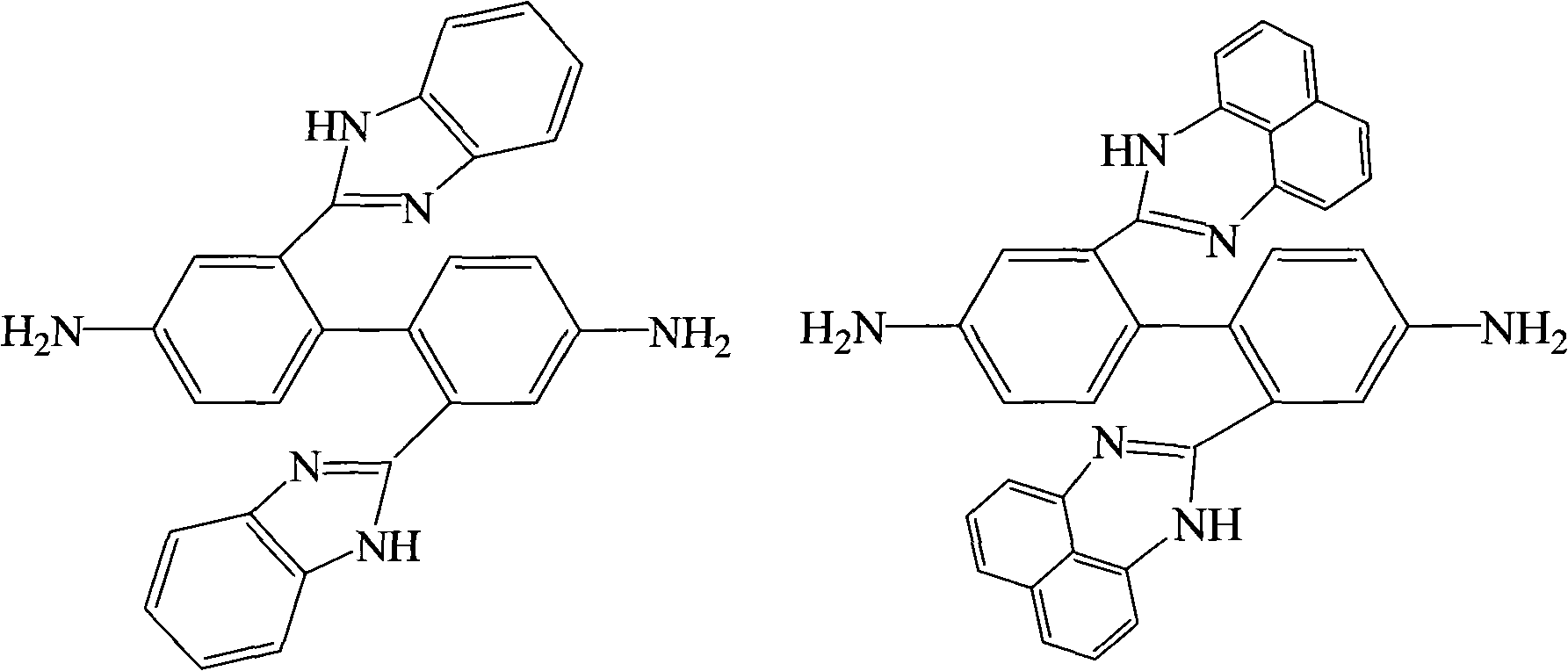
Side imidazolyl-containing benzidine derivatives and preparation method thereof
A technology of base benzidine and side imidazole, which is applied in the field of side imidazole group-containing benzidine derivatives and their preparation, can solve the problems of restricting the development of imidazole group-containing polymer varieties, few types of imidazole group-containing diamines, and the like, and achieves a rich variety. , good hydrolysis stability, the effect of improving performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] First, under ice bath conditions, 2.42 g (10 mmol) of raw material 2,2'-dicarboxybiphenyl was dissolved in 25 ml of concentrated sulfuric acid with a mass concentration of 98%, and then 1.4 ml (20.4 mmol) was added dropwise to the solution. ) concentrated nitric acid with a mass concentration of 65%. After the dropwise addition, the reaction condition was maintained at 0°C-5°C, and the reaction was stirred for 10 hours. After the reaction, slowly inject the reaction solution into crushed ice to obtain a yellow solid, which is repeatedly filtered and washed with water to weak acidity, and the solid is collected. The initial product is mixed with ethanol and water (the volume ratio of ethanol / water is 3 / 7-4 / 6) 90 milliliters of mixed solvents were recrystallized to obtain pure 2,2'-dicarboxy 4,4'-dinitrobiphenyl, which was collected by filtration and vacuum-dried for 6 hours at 50°C. The product quality was 2.2 grams, and the yield was 65 %.
[0024] Secondly, under the...
Embodiment 2
[0030]First, under ice-bath conditions, 2.42 grams (10 mmol) of raw material 2,2'-dicarboxybiphenyl was dissolved in 12 milliliters of concentrated sulfuric acid with a mass concentration of 98%, and then 2.1 milliliters (30.6 mmol) were added dropwise to the solution. ) concentrated nitric acid with a mass concentration of 65%. After the dropwise addition, the reaction condition was maintained at 0°C-5°C, and the reaction was stirred for 10 hours. After the reaction, slowly inject the reaction solution into crushed ice to obtain a yellow solid, which is repeatedly filtered and washed with water to weak acidity, and the solid is collected. The initial product is mixed with ethanol and water (the volume ratio of ethanol / water is 3 / 7-4 / 6) 90 milliliters of mixed solvents were recrystallized to obtain pure 2,2'-dicarboxy 4,4'-dinitrobiphenyl, which was collected by filtration and vacuum-dried for 6 hours at 50°C. The product quality was 2.5 grams, and the yield was 74 %.
[003...
Embodiment 3
[0035] First, under ice bath conditions, 2.42 grams (10 mmol) of raw material 2,2'-dicarboxybiphenyl was dissolved in 8 milliliters of concentrated sulfuric acid with a mass concentration of 98%, and then 1.4 milliliters (20.4 mmol) was added dropwise to the solution. ) concentrated nitric acid with a mass concentration of 65%. After the dropwise addition, the reaction condition was maintained at 0°C-5°C, and the reaction was stirred for 10 hours. After the reaction, slowly inject the reaction solution into crushed ice to obtain a yellow solid, which is repeatedly filtered and washed with water to weak acidity, and the solid is collected. The initial product is mixed with ethanol and water (the volume ratio of ethanol / water is 3 / 7-4 / 6) 90 milliliters of mixed solvents were recrystallized to obtain pure 2,2'-dicarboxy 4,4'-dinitrobiphenyl, which was collected by filtration and vacuum-dried for 6 hours at 50°C. The product quality was 2.3 grams, and the yield was 68 %.
[0036...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com