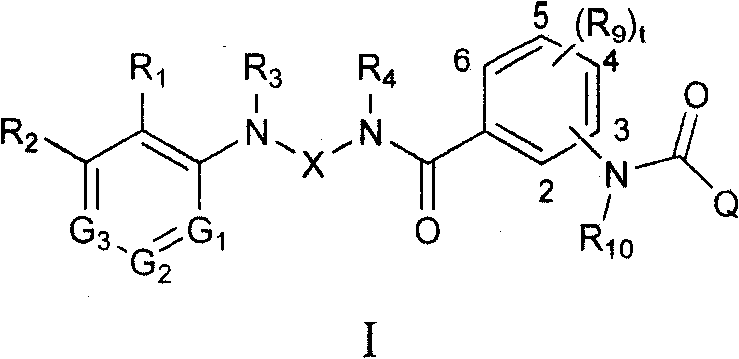
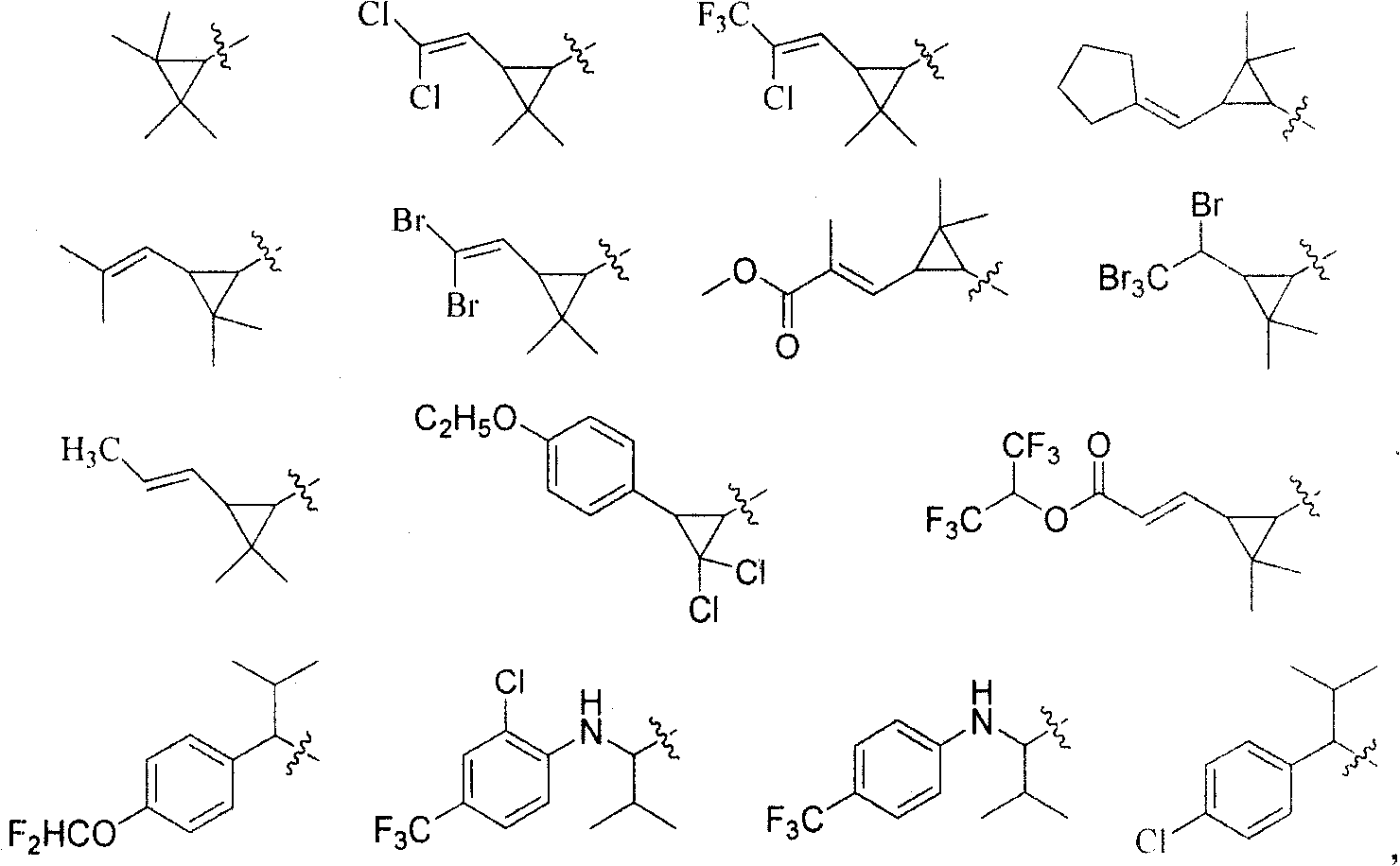
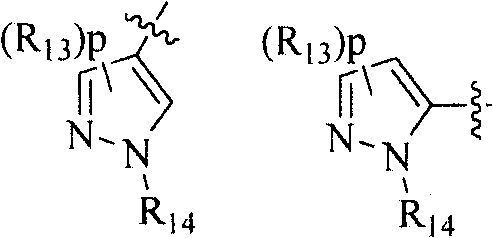Amide-type compound as well as preparation method and application thereof
An amide compound, C1-C6 technology, applied in the field of agricultural insecticides and fungicides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0454] Example 1: Preparation of Table 181-83 Compounds
[0455] (1) Preparation of Intermediate IV-1
[0456]
[0457] Add 9 grams (150 millimoles) of ethylenediamine and 100 milliliters of ethanol successively in a 250 milliliter reaction flask, then slowly drop 22.7 grams (100 millimoles) of 2,3-dichloro-5-trifluoromethylpyridine Add, add dropwise for 30 minutes, and react at room temperature for 2 hours. After the completion of the reaction as monitored by TLC, 22 g of light yellow oil was obtained by desolvation under reduced pressure. Yield 91.7%.
[0458] (2) Preparation of Table 181-83 Compounds
[0459]
[0460] 6-chloro-2-(3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazol-5-yl)-8-methyl-4H-3,1-benzoxazine- Add 1.00 g (2.21 mmol) of 4-ketone (see WO03015519 for preparation) into a 50 ml reaction flask, add 25 ml of acetonitrile, add 0.53 g of IV-1 (2.21 mmol) while stirring, and react at 40°C for 4 hours. After the completion of the TLC monitoring reaction, desol...
example 2
[0461] Example 2: Preparation of Table 181-287 Compounds
[0462] (1) Preparation of Intermediate IV-2
[0463]
[0464] Add 11.8 g (60 mmol) of piperazine (hexahydrate) into a 150 ml reaction flask containing 40 ml of acetonitrile, raise the temperature to 40°C, and add 2,3-dichloro-5-trifluoromethane dropwise under stirring 10.8 g (50 mmol) of pyridine was added within 15 minutes, then 9 ml of triethylamine was added, and the reaction was maintained at 40° C. for 4 hours. After the reaction was monitored by TLC, it was filtered and washed with a small amount of ethanol to obtain 12 g of a white solid. Yield 90.2%.
[0465] (2) Preparation of Table 181-287 Compounds
[0466]
[0467] 6-chloro-2-(3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazol-5-yl)-8-methyl-4H-3,1-benzoxazine- Add 1.00 g (2.21 mmol) of 4-ketone (see WO03015519 for preparation) into a 50 ml reaction flask, add 25 ml of acetonitrile, add 0.59 g of IV-2 (2.21 mmol) while stirring, and react at 40°C for 4 h...
example 3
[0473] Example 3: 60% WP
[0474] Table 181-288 Active ingredient 60%
[0475] Sodium dodecyl naphthalene sulfonate 2%
[0476] Sodium lignosulfonate 9%
[0477] Kaolin topped up to 100%
[0478] The components (all solids) were mixed together and pulverized in a pulverizer until the granules were on par.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com