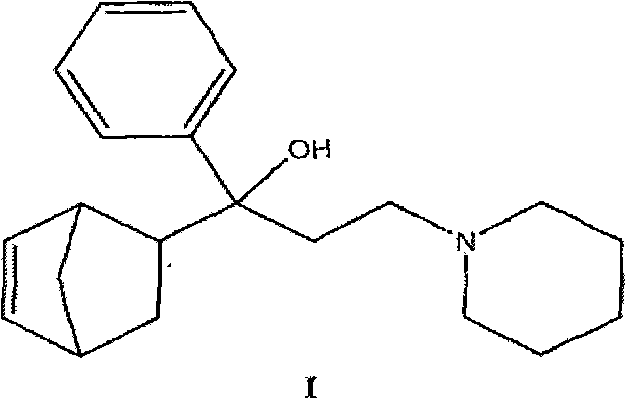
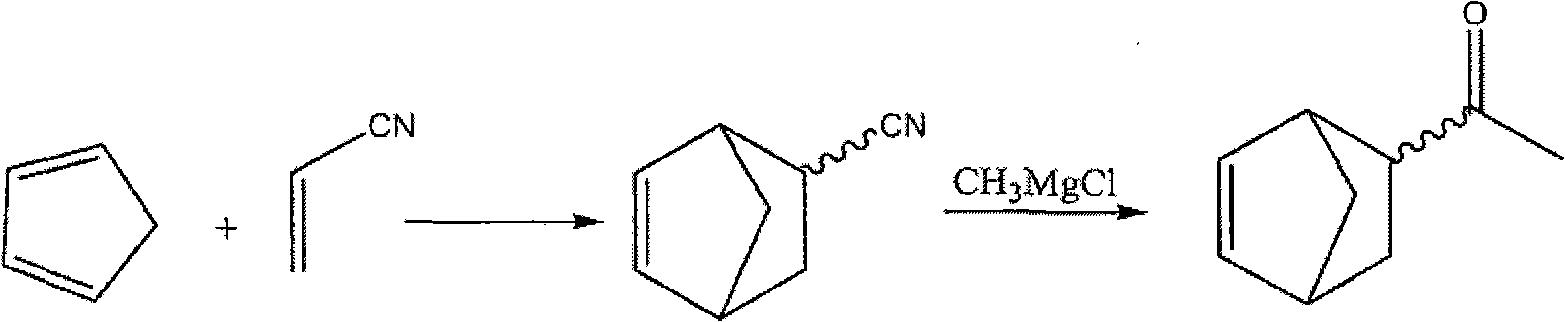
A process for producing acetylnorbornene, an intermediate in the synthesis of biperiden
A kind of norbornene and acetyl technology, which is used in the field of preparing the intermediate acetyl norbornene used in the synthesis of biperiden, and can solve the problems of high toxicity, difficulty in handling, and inability to fully satisfy industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Preparation of 5-Cyanonorbornene
[0018] To a solution of acrylonitrile (249.4 g, 4.7 moles) in methanol (260 mL) was slowly added cyclopentadiene monomer (311.0 g, 4.7 moles) over 45 minutes at 40°C. The reaction temperature was maintained at 40 to 50°C for 1 hour. The solvent was removed under vacuum to give a residue (487.3 g), which was distilled under high vacuum to give 5-cyanonorbornene (453.3 g, 80.8% yield).
Embodiment 2
[0020] Preparation of 5-acetyl norbornene
[0021] 1,4-Dioxane was slowly added to a solution of methylmagnesium chloride (263.6 mL, 3.0 M in THF, 0.53 mol) at room temperature over 1 hour. 5-Cyanonorbornene (30 g, 0.25 mol) was then added slowly at room temperature over 1 hour and the reaction mixture was refluxed for 90 minutes. After the reaction was complete, the reaction mixture was cooled and poured into ice-cold water (200 mL). Acetylnorbornene (25.5 g, 75% yield) prepared by vacuum distillation was routinely tested. The ratio of exo to endo isomer was 1.8 [gas chromatography analysis (area %): exo / endo=41.80 / 23.16=1.8].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com