Multi-functional small molecules as anti-proliferative agents
A technology for multifunctional, small molecule compounds, applied in the field of multifunctional small molecules as anti-proliferative preparations, which can solve the problems of destruction of the efficacy of pharmaceutical compositions, high health care costs, and limited effectiveness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
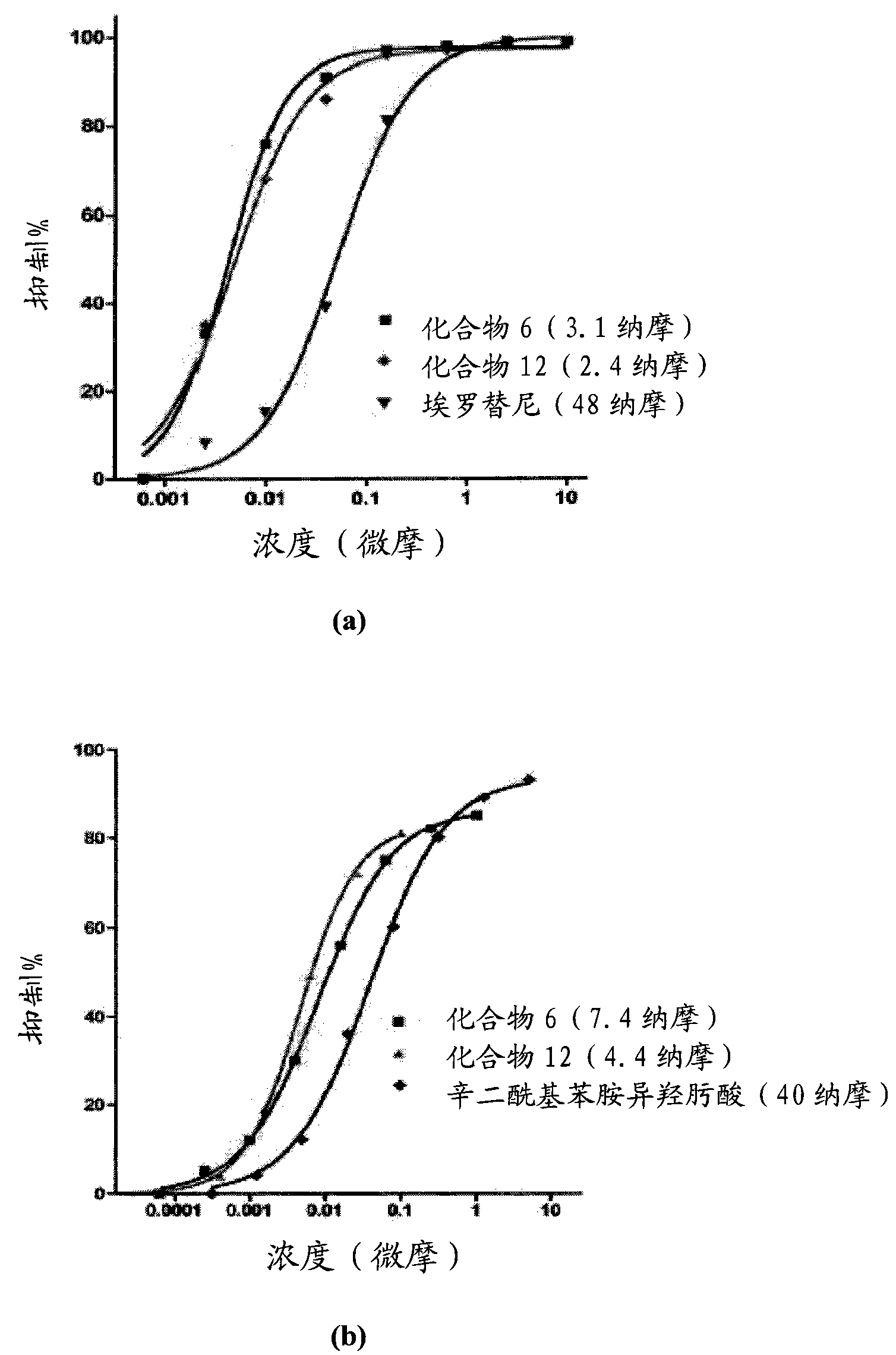
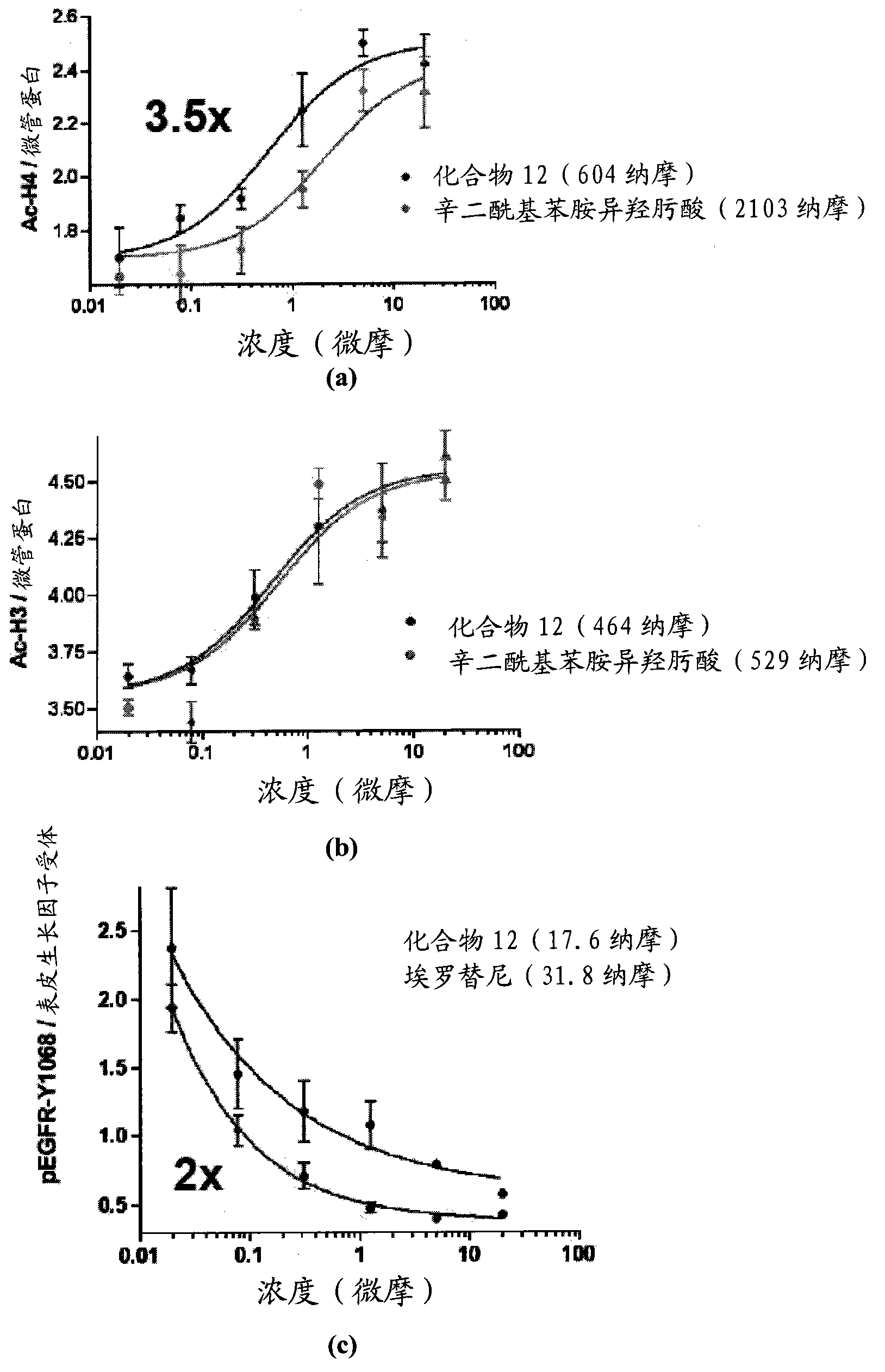
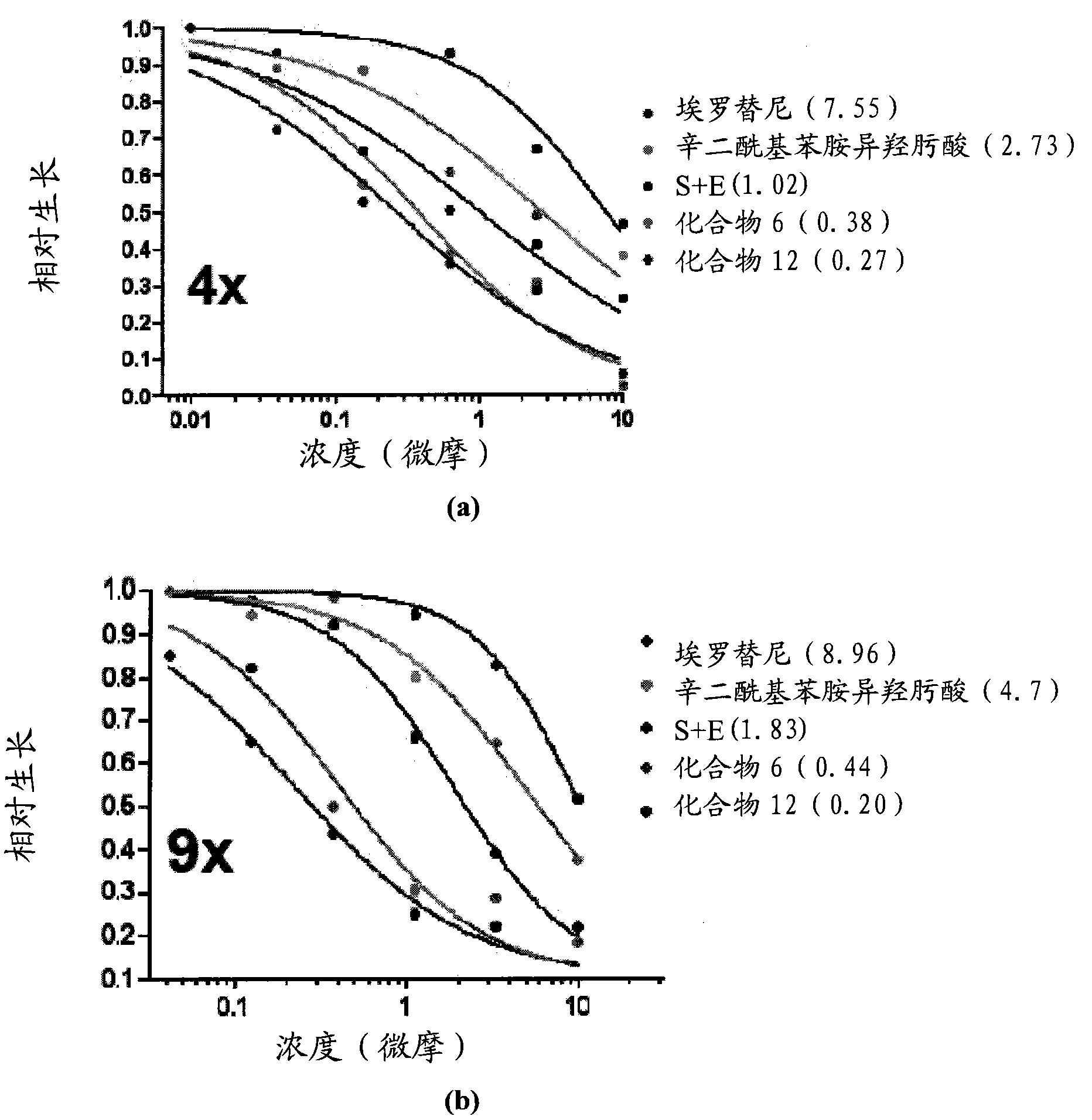
Image
Examples
preparation example Construction
[0296] Methods of preparing pharmaceutical compositions containing active ingredients are well known in the art, for example, by mixing, granulating, or tabletting methods. The active therapeutic ingredient is usually mixed with a pharmaceutically acceptable carrier, and the pharmaceutically acceptable carrier is compatible with the active ingredient. For oral administration, the active formulation is mixed with additives customary for oral purposes, such as vehicles, stabilizers, or inert diluents, and converted by conventional methods into a suitable dosage form. Forms such as tablets, coated tablets, hard or soft gelatin capsules, aqueous, alcoholic or oily solutions, and the like as detailed above.
[0297] The dosage of the compounds of the invention administered to the patient is below that which would induce toxicity in the patient. In certain embodiments, the dose of the compound administered to the patient is less than the dose that results in the concentration of th...
Embodiment 1
[0432] Example 1: 2-(4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolinyl-6- Oxygen)-N-hydroxyacetamide (compound 1) preparation method
[0433] Step 1a. 6,7-Dimethoxyquinazolinyl-4(3hydro)-one (Compound 0102)
[0434] A mixture of methyl 2-amino-4,5-dimethoxybenzoate 0101 (2.1 g, 10 mmol), ammonium formate (0.63 g, 10 mmol) and formamide (7 ml) was stirred, And heated to 190-200°C for 2 hours. The mixture was then cooled to room temperature. The resulting precipitate was isolated, washed with water and dried to afford the title compound 0102 (1.8 g, 84.7%) as a brown solid: LCMS: m / z 207 [M+1] + ; 1 H NMR (dimethylsulfoxide.) δ3.87(s, 3H), 3.89(s, 3H), 7.12(s, 1H), 7.43(s, 1H), 7.97(s, 1H), 12.08(bs , 1H).
[0435] Step 1b. 6-Hydroxy-7-methoxyquinazolinyl-4(3hydro)-one (Compound 0103)
[0436] To stirred methanesulfonic acid (68 mL) was added 6,7-dimethoxyquinazolinyl-4(3hydro)-one (0102) (10.3 g, 50 mmol) in portions. Subsequently, L-methionine (8.6 g, 57.5 mmol) was ...
Embodiment 2
[0450] Example 2: 4-(4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolinyl-6- The preparation method of oxy)-N-hydroxybutyramide (compound 3)
[0451] Step 2a. Ethyl 4-(4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolinyl-6-oxy)butanoate (Compound 0110-3)
[0452] The title compound 0110-3 was prepared as a yellow solid (220 mg, 80.5%) from compound 0109 (200 mg, 0.63 mmol) obtained in step 1f and ethyl 4-bromobutyrate (135 mg, 0.69 mmol) prepared by a method similar to that described in the preparation method of compound 0110-1 (Example 1): LCMS: m / z 434 [M+1] + ; 1 H NMR (deuterated chloroform.) δ1.36(t, 3H), 2.23(m, 2H), 2.57(t, 2H), 4.03(s, 3H), 4.32(m, 4H), 7.15(t, 1H ), 7.25 (m, 1H), 7.87 (s, 1H), 8.00 (m, 2H), 8.15 (bs, 1H), 8.57 (s, 1H).
[0453] Step 2b. 4-(4-(3-Chloro-4-fluoroanilino)-7-methoxyquinazolinyl-6-oxyl)-N-hydroxybutanamide (Compound 3)
[0454] The title compound 3 was prepared as a gray solid (25 mg, 12%) from compound 0110-3 (200 mg, 0.23 mmol) as des...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com