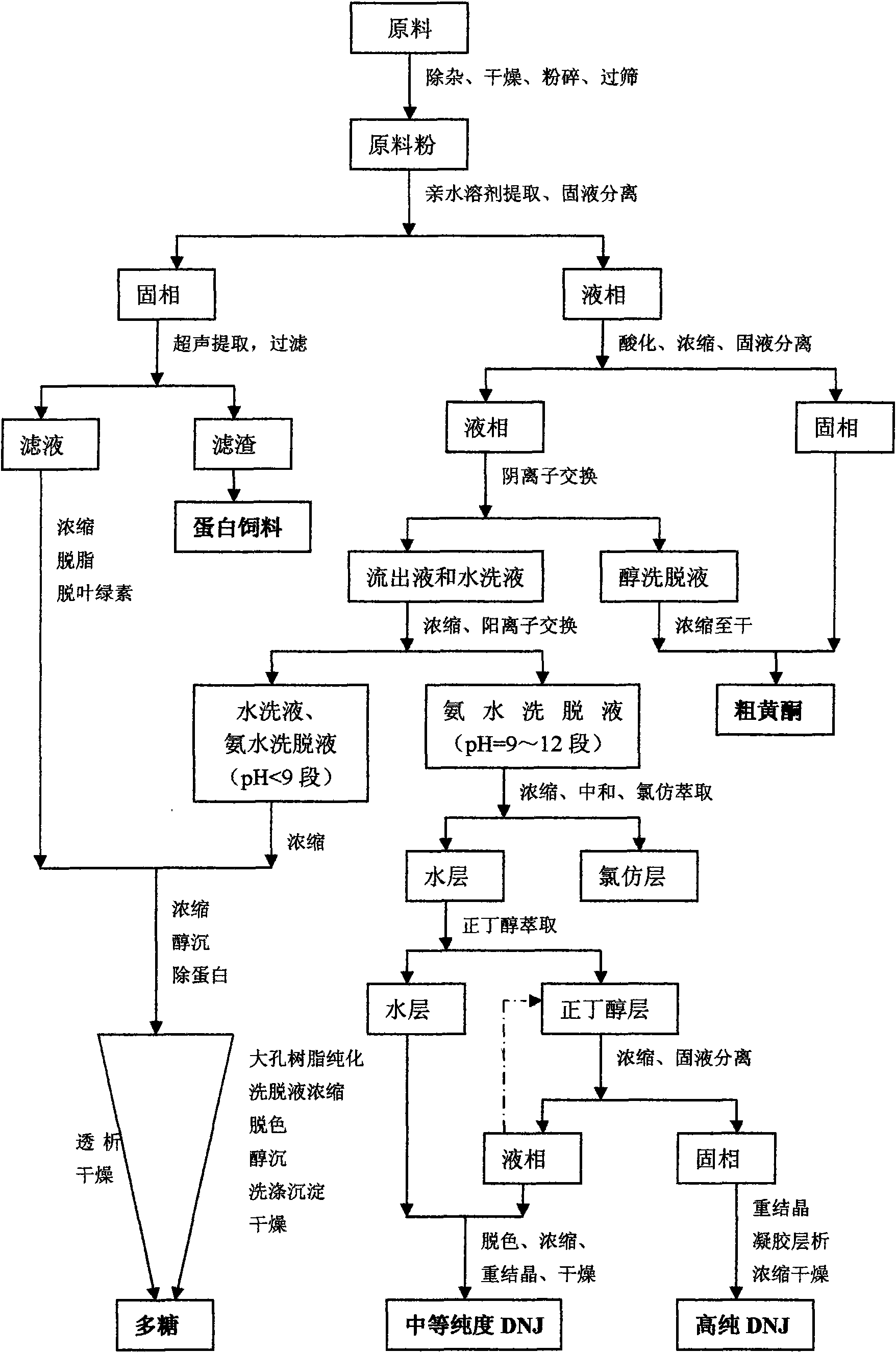
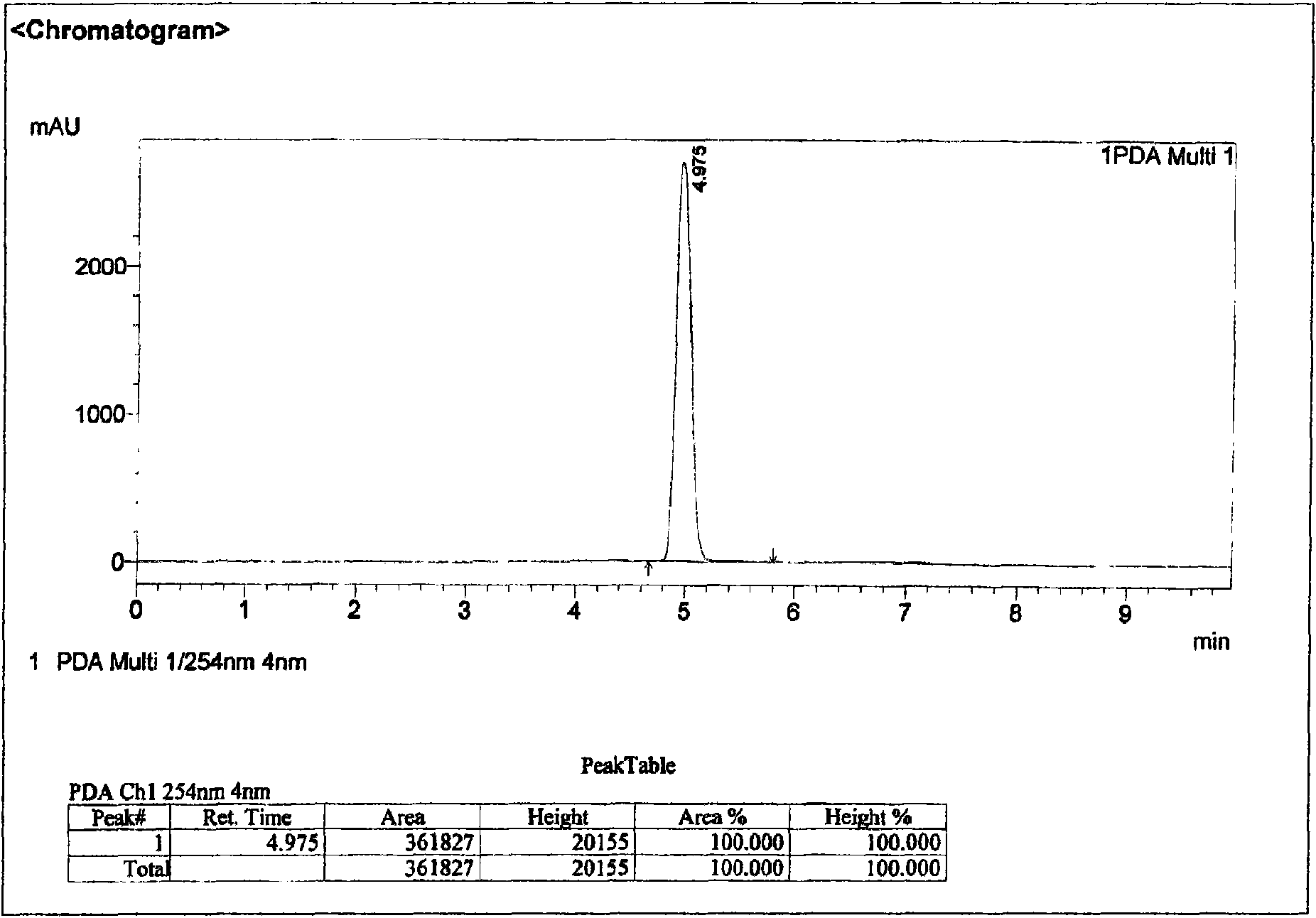
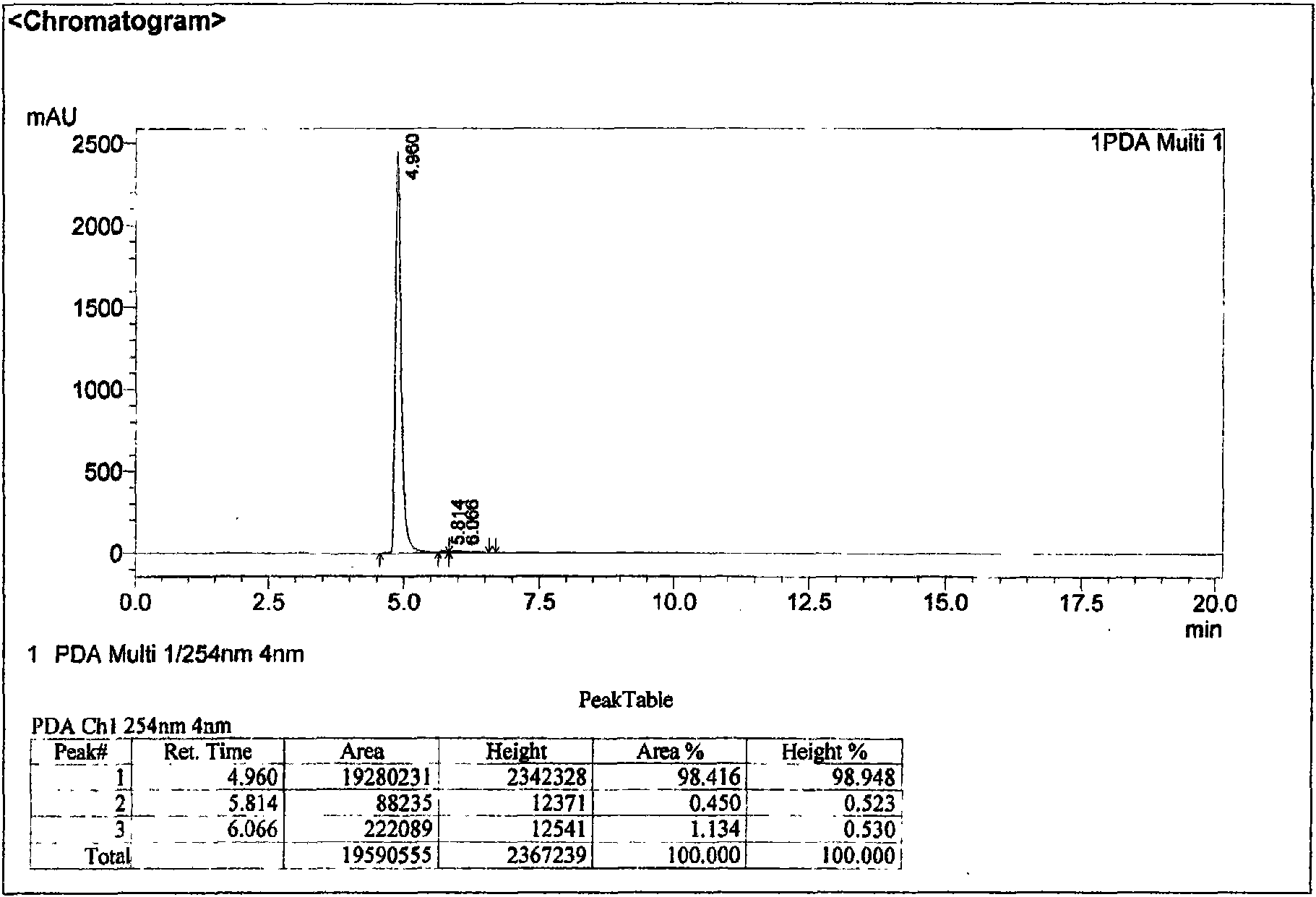
Method for extracting and separating 1-deoxynojirimycin with high purity from natural products
A technology of deoxynojirimycin and natural products, which is applied in the field of traditional Chinese medicine, and achieves the effects of mature technology, low equipment cost and obvious economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Extraction of high-purity 1-deoxynojirimycin and preparation of by-products from mulberry leaves
[0046] A. Extraction and separation of high-purity 1-deoxynojirimycin:
[0047]The mulberry leaves are washed and dried, crushed, and passed through a 40-mesh sieve. Weigh 50kg of mulberry leaf powder, add 400L 65% ethanol, ultrasonically extract at 60°C 800HZ for 30min, filter, add 200L 65% ethanol to the filter residue, extract ultrasonically at 60°C 800HZ for 30min, filter, combine the filtrates, and filter the residue for use.
[0048] Add 2 mol / L hydrochloric acid to the filtrate in 1), adjust pH=2, concentrate under reduced pressure to 20 L, filter with suction, and filter residue for use. Add the filtrate to 100kg717 anion exchange resin, stir for 15min, let it sit for 6h, then release the liquid, add the effluent to the original anion resin, repeat 3 times, add 300L water to elute, collect the effluent and washing water, and concentrate to 20L.
[0049]...
Embodiment 2
[0058] Example 2 Extraction of high-purity 1-deoxynojirimycin and preparation of by-products from silkworm excrement
[0059] A. Extraction and separation of high-purity 1-deoxynojirimycin:
[0060] 1) Take the 5th instar silkworm excrement, dry it, crush it, and pass it through a 40-mesh sieve. Weigh 400g of silkworm excrement powder, add 4000mL 65% ethanol, ultrasonically extract at 60°C 1500HZ for 25min, filter, add 2400mL 65% ethanol to the filter residue, extract ultrasonically at 60°C 1500HZ for 25min, filter, combine the filtrates, and filter the residue for use.
[0061] Add 2 mol / L hydrochloric acid to the filtrate in 1), adjust pH=3, concentrate under reduced pressure to 200 mL, filter with suction, and filter the residue for use. Add the filtrate to 1kg717 anion exchange resin, stir for 15min, let it sit for 6h, then release the liquid, add the effluent to the original anion resin, repeat 3 times, slowly add 3L water to elute, collect the third effluent and washing...
Embodiment 3
[0071] Embodiment 3 extracts high-purity 1-deoxynojirimycin from mulberry fruit:
[0072] 1) The mulberries are dried and pulverized, and passed through a 40-mesh sieve. Weigh 2kg of mulberry powder, add 15L 80% methanol, 70°C 1200HZ ultrasonic extraction for 20min, filter, add 10L 80% methanol to the filter residue, 70°C 1200HZ ultrasonic extraction for 20min, filter, combine the filtrates, and filter residue for use.
[0073] 2) Add 2 mol / L sulfuric acid to the filtrate in 1), adjust pH=3, concentrate under reduced pressure to 1 L, filter with suction, and filter the residue for use. Add the filtrate to 5kg701 anion exchange resin, stir evenly, let the liquid release after 10 hours of stuffing, add the effluent to the original anion resin, repeat 3 times, add 20L water to elute, collect the third effluent and washing water, and concentrate to 1L.
[0074] Purify the concentrated solution in 2) by 5kg732 cation exchange resin column chromatography: after loading the sample, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com